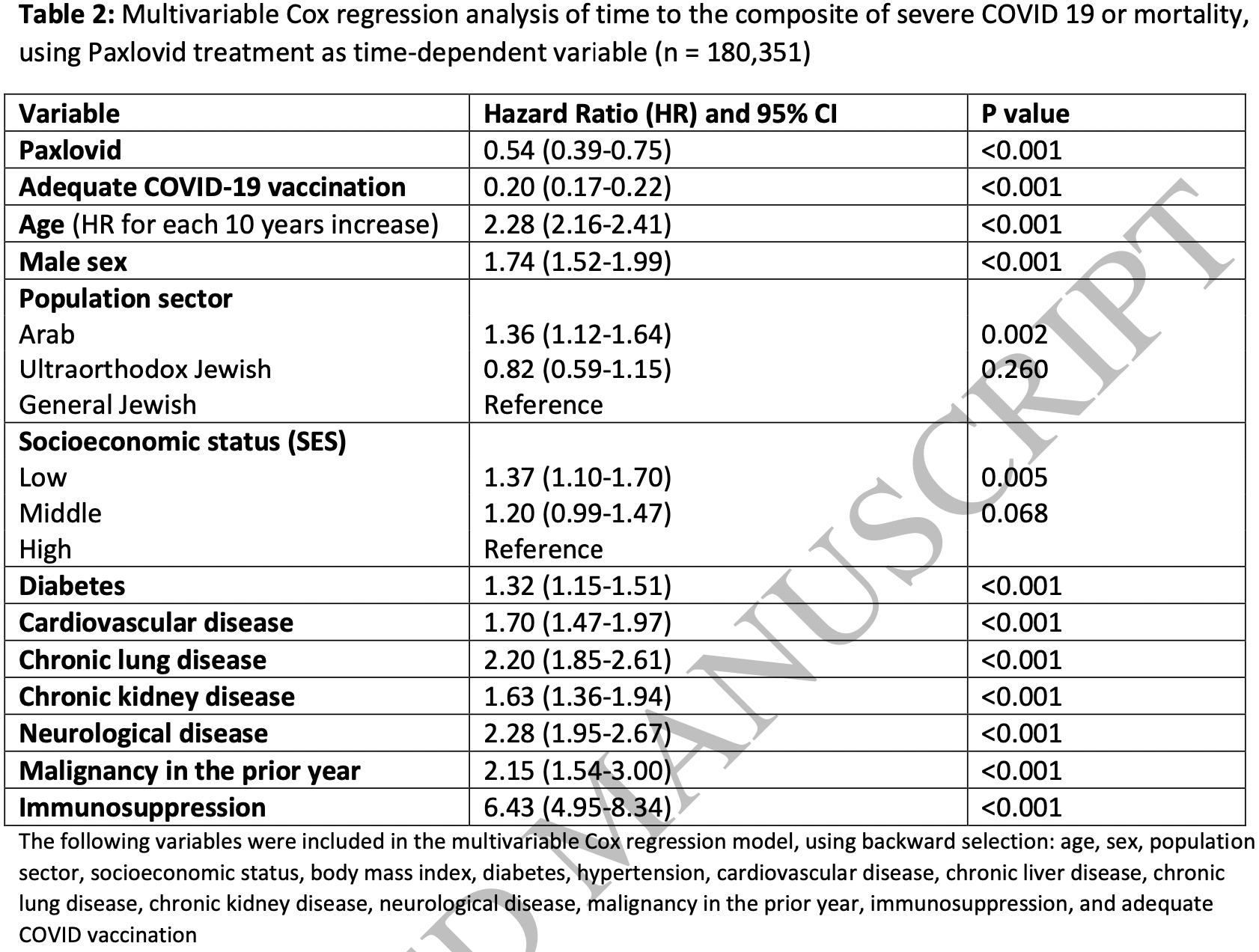
Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac443, Jun 2022
Retrospective 180,351 patients in Israel, 4,737 treated with paxlovid, showing significantly lower combined severe COVID-19 / mortality with treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
|
risk of severe case or mortality, 46.0% lower, HR 0.54, p < 0.001, treatment 4,737, control 175,614, adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Najjar-Debbiny et al., 2 Jun 2022, retrospective, Israel, peer-reviewed, 8 authors, study period 1 January, 2022 - 28 February, 2022.
Contact: ronzana@clalit.org.il, ronza.najjar@gmail.com.
Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients
doi:10.1093/cid/ciac443/6599020
Background: Paxlovid was granted emergency use authorization for the treatment of mild to moderate COVID-19, based on the interim analysis of EPIC-HR trial. Paxlovid effectiveness needs to be assessed in a noncontrolled setting. In this study we used population-based real world data to evaluate the effectiveness of Paxlovid. Methods: The database of the largest healthcare provider in Israel was used to identify all adults aged 18 years or older with first ever positive test for SARS-CoV-2 between January and February 2022, who were at high risk for severe COVID-19 and had no contraindications for Paxlovid use. Patients were included irrespective of their COVID-19 vaccination status. Cox hazard regression was used to estimate the 28 day HR for severe COVID-19 or mortality with Paxlovid examined as time-dependent variable. Results: Overall, 180,351 eligible were included, of them only 4,737 (2.6%) were treated with Paxlovid, and 135,482 (75.1%) had adequate COVID-19 vaccination status. Both Paxlovid and adequate COVID-19 vaccination status were associated with significant decrease in the rate of severe COVID-19 or mortality with adjusted HR 0.54 (95% CI, 0.39-0.75) and 0.20 (95% CI, 0.17-0.22), respectively. Paxlovid appears to be more effective in older patients, immunosuppressed patients, and patients with underlying neurological or cardiovascular disease (interaction p-value <0.05 for all). No significant interaction was detected between Paxlovid treatment and COVID-19 vaccination status. Conclusions: This study suggests that in the era of omicron and in real life setting Paxlovid is highly effective in reducing the risk of severe COVID-19 or mortality.
All data relevant to this analysis were presented in the paper.
References
Arbel, Hammerman, Sergienko, BNT162b2 Vaccine Booster and Mortality Due to Covid-19, N Engl J Med
Bajema, Dahl, Evener, Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans -Five Veterans
Barda, Dagan, Ben-Shlomo, Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting, N Engl J Med
Drożdżal, Rosik, Lechowicz, An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment, Drug Resist Updat, doi:10.1016/j.drup.2021.100794
Food, Emergency Use Authorization 105
Hammond, Leister-Tebbe, Gardner, EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Madhi, Kwatra, Myers, Population Immunity and Covid-19 Severity with Omicron Variant in South Africa, N Engl J Med, doi:10.1056/NEJMoa2119658
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ, doi:10.1136/bmj.n2713
Parums, Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients, Med Sci Monit
Pfizer, Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death
Rosenberg, Holtgrave, New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status
Tenforde, Self, Naioti, Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults -United States, March, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7034e2
Weinreich, Sivapalasingam, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
DOI record:
{
"DOI": "10.1093/cid/ciac443",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac443",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Paxlovid was granted emergency use authorization for the treatment of mild to moderate COVID-19, based on the interim analysis of EPIC-HR trial. Paxlovid effectiveness needs to be assessed in a noncontrolled setting. In this study we used population-based real world data to evaluate the effectiveness of Paxlovid.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The database of the largest healthcare provider in Israel was used to identify all adults aged 18 years or older with first ever positive test for SARS-CoV-2 between January and February 2022, who were at high risk for severe COVID-19 and had no contraindications for Paxlovid use. Patients were included irrespective of their COVID-19 vaccination status. Cox hazard regression was used to estimate the 28 day HR for severe COVID-19 or mortality with Paxlovid examined as time-dependent variable.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Overall, 180,351 eligible were included, of them only 4,737 (2.6%) were treated with Paxlovid, and 135,482 (75.1%) had adequate COVID-19 vaccination status. Both Paxlovid and adequate COVID-19 vaccination status were associated with significant decrease in the rate of severe COVID-19 or mortality with adjusted HR 0.54 (95% CI, 0.39-0.75) and 0.20 (95% CI, 0.17-0.22), respectively. Paxlovid appears to be more effective in older patients, immunosuppressed patients, and patients with underlying neurological or cardiovascular disease (interaction p-value &lt;0.05 for all). No significant interaction was detected between Paxlovid treatment and COVID-19 vaccination status.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>This study suggests that in the era of omicron and in real life setting Paxlovid is highly effective in reducing the risk of severe COVID-19 or mortality.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7586-3714",
"affiliation": [
{
"name": "Lady Davis Carmel Medical Center Infection Control and Prevention Unit, , Haifa, Israel"
},
{
"name": "Technion-Israel Institute of Technology Ruth and Bruce Rappaport Faculty of Medicine, , Haifa, Israel"
}
],
"authenticated-orcid": false,
"family": "Najjar-Debbiny",
"given": "Ronza",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Technion-Israel Institute of Technology Ruth and Bruce Rappaport Faculty of Medicine, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Department of Community Medicine and Epidemiology, , Haifa, Israel"
}
],
"family": "Gronich",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Technion-Israel Institute of Technology Ruth and Bruce Rappaport Faculty of Medicine, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Infectious Diseases unit, , Haifa, Israel"
}
],
"family": "Weber",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lady Davis Carmel Medical Center Pulmonology Division, , Haifa, Israel"
},
{
"name": "Yale school of Medicine Pulmonology, Critical Care and Sleep Medicine, , New Haven, Connecticut, USA"
}
],
"family": "Khoury",
"given": "Johad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lady Davis Carmel Medical Center Infectious Diseases unit, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Internal Medicine C, , Haifa, Israel"
}
],
"family": "Amar",
"given": "Maisam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lady Davis Carmel Medical Center Department of Community Medicine and Epidemiology, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Statistical Unit, , Haifa, Israel"
}
],
"family": "Stein",
"given": "Nili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Technion-Israel Institute of Technology Ruth and Bruce Rappaport Faculty of Medicine, , Haifa, Israel"
},
{
"name": "Emek Medical Center Internal Medicine C, , Afula, Israel"
},
{
"name": "Emek Medical Center Clinical Pharmacology Unit, , Afula, Israel"
}
],
"family": "Goldstein",
"given": "Lee Hilary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Technion-Israel Institute of Technology Ruth and Bruce Rappaport Faculty of Medicine, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Department of Community Medicine and Epidemiology, , Haifa, Israel"
},
{
"name": "Lady Davis Carmel Medical Center Translational Epidemiology Unit and Research Authority, , Haifa, Israel"
}
],
"family": "Saliba",
"given": "Walid",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
2
]
],
"date-time": "2022-06-02T17:45:42Z",
"timestamp": 1654191942000
},
"deposited": {
"date-parts": [
[
2022,
6,
2
]
],
"date-time": "2022-06-02T17:45:43Z",
"timestamp": 1654191943000
},
"indexed": {
"date-parts": [
[
2022,
6,
2
]
],
"date-time": "2022-06-02T18:13:58Z",
"timestamp": 1654193638638
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
6,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
2
]
],
"date-time": "2022-06-02T00:00:00Z",
"timestamp": 1654128000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac443/43934598/ciac443.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac443/43934598/ciac443.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
6,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac443/6599020"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients",
"type": "journal-article"
}