Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac687, May 2022 (preprint)
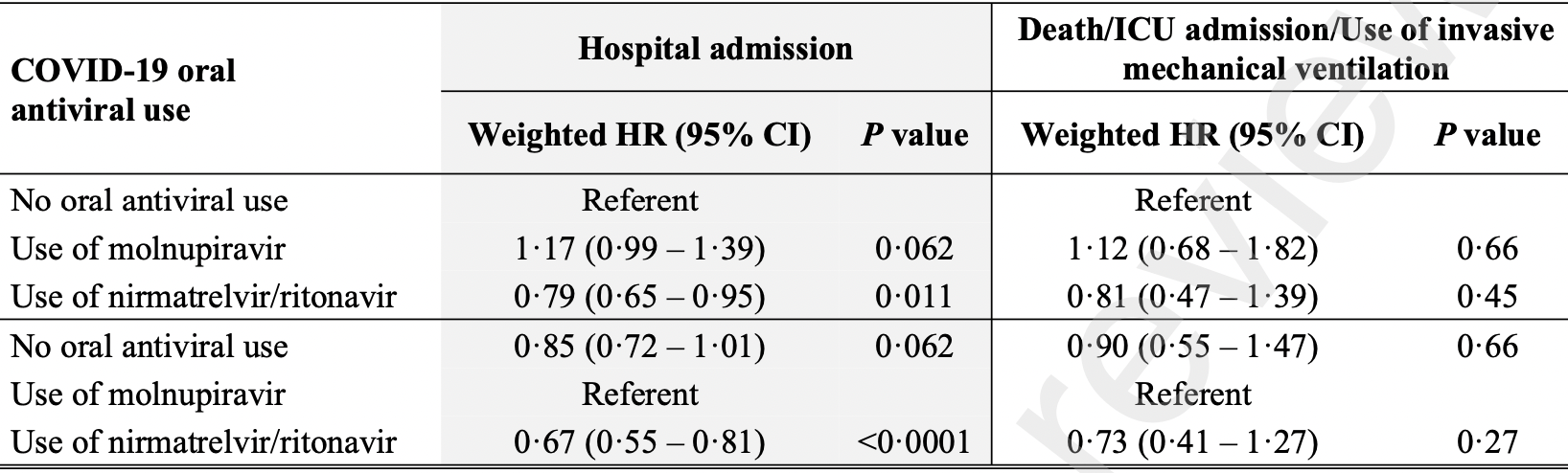
Propensity score weighted retrospective of 93,883 outpatients in Hong Kong, 5,808 treated with molnupiravir and 4,921 treated with paxlovid, showing higher hospitalization and higher combined mortality/mechanical ventilation/ICU admission with molnupiravir, without statistical significance; and lower hospitalization and combined mortality/mechanical ventilation/ICU admission with paxlovid, statistically significant only for hospitalization.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
Study covers paxlovid and molnupiravir.
|
combined death/ventilation/ICU, 19.0% lower, HR 0.81, p = 0.45, treatment 23 of 4,921 (0.5%), control 151 of 83,154 (0.2%), propensity score weighting, Cox proportional hazards, day 30.
|
|
risk of hospitalization, 21.0% lower, HR 0.79, p = 0.01, treatment 172 of 4,921 (3.5%), control 1,322 of 83,154 (1.6%), propensity score weighting, Cox proportional hazards, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Yip et al., 24 May 2022, retrospective, placebo-controlled, China, peer-reviewed, 11 authors, study period 16 February, 2022 - 31 March, 2022.
Contact: wonglaihung@cuhk.edu.hk.
Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients
References
Ahmad, Batool, Ain, Kim, Choi, Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations, Int J Mol Sci
Balint, Voros-Horvath, Szechenyi, Omicron: increased transmissibility and decreased pathogenicity, Signal Transduct Target Ther
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Clin Infect Dis
Bloomberg, Hong Kong's Nursing Homes Are Unvaccinated Hotbeds of Covid
Cheung, Fung, Chow, Tung, Structured data entry of clinical information for documentation and data collection, Stud Health Technol Inform
Consortium, Whost, Pan, Peto, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Dal-Re, Becker, Bottieau, Holm, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis
De Anda, Johnson, Pedley, Molnupiravir for Covid-19 in Nonhospitalized Patients. Reply, N Engl J Med
Gandhi, Malani, Rio, COVID-19 Therapeutics for Nonhospitalized Patients, JAMA
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hksar Government, Overall first-dose COVID-19 vaccination rate of RCHEs and RCHDs rises to 84 per cent on 11
Hui, Zumla, Advances in the epidemiology, clinical features, diagnosis, clinical management and prevention of coronavirus disease 2019, Curr Opin Pulm Med
Jo, Kim, Radnaabaatar, Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea, Epidemiol Health
Kabinger, Stiller, Schmitzova, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Krammer, SARS-CoV-2 vaccines in development, Nature
Lui, Yip, Wong, Significantly Lower Case-fatality Ratio of Coronavirus Disease 2019 (COVID-19) than Severe Acute Respiratory Syndrome (SARS) in Hong Kong-A Territory-Wide Cohort Study, Clin Infect Dis
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis
Pfizer, Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR
Roth, Emmons-Bell, Alger, Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States During the COVID-19 Pandemic, JAMA Netw Open
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs, Clin Infect Dis
Thorlund, Sheldrick, Meyerowitz-Katz, Singh, Hill, Making Statistical Sense of the Molnupiravir MOVe-OUT Clinical Trial, Am J Trop Med Hyg
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Wong, Au, Lau, Lau, Cowling et al., community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study, doi:10.1101/2022.05.26.22275631
Yip, Wong, Lui, Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19, Hepatology
DOI record:
{
"DOI": "10.1093/cid/ciac687",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac687",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>We examined the effectiveness of molnupiravir and nirmatrelvir/ritonavir in reducing hospitalization and deaths in a real-world cohort of non-hospitalized COVID-19 patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was a territory-wide retrospective cohort study in Hong Kong. Non-hospitalized COVID-19 patients who attended designated outpatient clinics between 16 February and 31 March 2022 were identified. Patients hospitalized on the day of the first clinic appointment or used both oral antivirals were excluded. The primary endpoint was hospitalization. The secondary endpoint was a composite of intensive care unit admission, invasive mechanical ventilation use, and/or death.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 93,883 patients, 83,154 (88.6%), 5,808 (6.2%), and 4,921 (5.2%) were oral antiviral non-users, molnupiravir users, and nirmatrelvir/ritonavir users respectively. Compared to non-users, oral antiviral users were older and had more comorbidities, lower complete vaccination rate, and more hospitalizations in the previous year. Molnupiravir users were older, and had more comorbidities, lower complete vaccination rate, and more hospitalizations in the previous year than nirmatrelvir/ritonavir users. At a median follow-up of 30 days, 1,931 (2.1%) patients were hospitalized and 225 (0.2%) patients developed the secondary endpoint. After propensity score weighting, nirmatrelvir/ritonavir use (weighted hazard ratio 0.79, 95%CI 0.65-0.95, P = 0.011) but not molnupiravir use (weighted hazard ratio 1.17, 95%CI 0.99-1.39, P = 0.062) was associated with a reduced risk of hospitalization than non-users. The use of molnupiravir or nirmatrelvir/ritonavir was not associated with a lower risk of the secondary endpoint as compared to non-users.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Use of nirmatrelvir/ritonavir but not molnupiravir was associated with a reduced risk of hospitalization in real-world non-hospitalized COVID-19 patients.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1819-2464",
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Institute of Digestive Disease"
}
],
"authenticated-orcid": false,
"family": "Yip",
"given": "Terry Cheuk Fung",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Stanley Ho Centre for Emerging Infectious Diseases, Jockey Club School of Public Health & Primary Care"
}
],
"family": "Lui",
"given": "Grace Chung Yan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
}
],
"family": "Lai",
"given": "Mandy Sze Man",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Stanley Ho Centre for Emerging Infectious Diseases, Jockey Club School of Public Health & Primary Care"
}
],
"family": "Wong",
"given": "Vincent Wai Sun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Institute of Digestive Disease"
}
],
"family": "Tse",
"given": "Yee Kit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
}
],
"family": "Ma",
"given": "Bosco Hon Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
}
],
"family": "Hui",
"given": "Elsie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine, Prince of Wales Hospital, Hospital Authority , Hong Kong"
}
],
"family": "Leung",
"given": "Maria KW",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Faculty of Medicine, The Chinese University of Hong Kong ; Hong Kong"
},
{
"name": "Department of Internal Medicine, Union Hospital , Hong Kong"
}
],
"family": "Chan",
"given": "Henry Lik Yuen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Stanley Ho Centre for Emerging Infectious Diseases, Jockey Club School of Public Health & Primary Care"
}
],
"family": "Hui",
"given": "David Shu Cheong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2863-9389",
"affiliation": [
{
"name": "Department of Medicine and Therapeutics"
},
{
"name": "Medical Data Analytics Centre (MDAC)"
},
{
"name": "Institute of Digestive Disease"
}
],
"authenticated-orcid": false,
"family": "Wong",
"given": "Grace Lai Hung",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T01:48:15Z",
"timestamp": 1661737695000
},
"deposited": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T01:48:16Z",
"timestamp": 1661737696000
},
"indexed": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T02:11:14Z",
"timestamp": 1661739074182
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T00:00:00Z",
"timestamp": 1661731200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac687/45601511/ciac687.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac687/45601511/ciac687.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
29
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac687/6678124"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients",
"type": "journal-article"
}
yip