Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality for At-risk COVID-19 Outpatients During an Omicron BA.1 and BA.1.1-Predominant Phase
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.10.002, Jun 2022 (preprint)
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
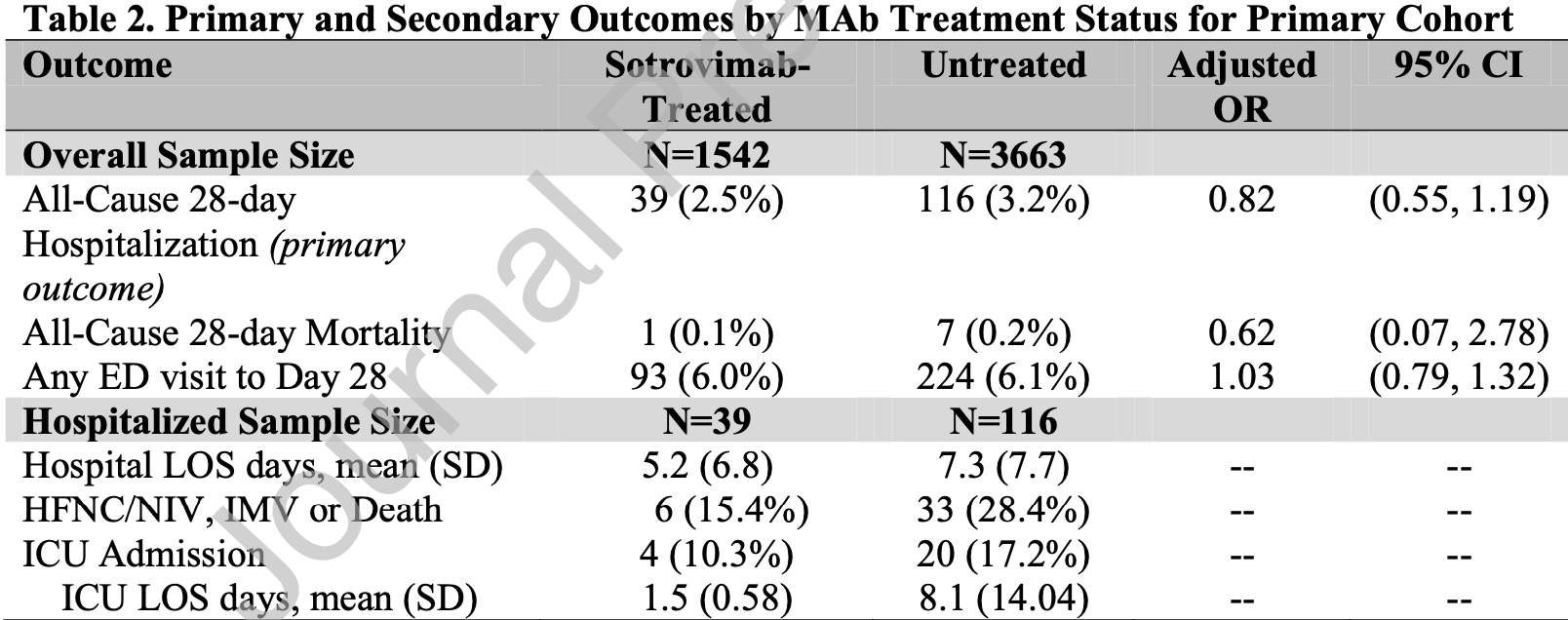
Retrospective 30,247 outpatients in the USA, showing no significant differences with sotrovimab with omicron BA.1.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Although the 38% lower mortality is not statistically significant, it is consistent with the significant 47% lower mortality [8‑69%] from meta-analysis of the 15 mortality results to date.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 38.0% lower, RR 0.62, p = 0.62, treatment 1 of 1,542 (0.1%), control 7 of 3,663 (0.2%), odds ratio converted to relative risk.
|
|
risk of hospitalization, 17.5% lower, RR 0.82, p = 0.32, treatment 39 of 1,542 (2.5%), control 116 of 3,663 (3.2%), NNT 157, odds ratio converted to relative risk, primary outcome.
|
|
risk of progression, 2.8% higher, RR 1.03, p = 0.83, treatment 93 of 1,542 (6.0%), control 224 of 3,663 (6.1%), NNT 1189, odds ratio converted to relative risk, ED visit.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Aggarwal et al., 18 Jun 2022, retrospective, USA, peer-reviewed, 10 authors, study period 26 December, 2021 - 10 March, 2022.
Contact: neil.aggarwal@cuanschutz.edu.
Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.10.002
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding)
References
Aggarwal, Beaty, Bennett, Carlson, Davis et al., Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients, J Infect Dis
Angus, Optimizing the Trade-off Between Learning and Doing in a Pandemic, JAMA
Bennett, Moffitt, Hajagos, Amor, Anand et al., Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative, JAMA Netw Open
Cameroni, Saliba, Bowen, Rosen, Culap et al., Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, bioRxiv
Chavarot, Melenotte, Amrouche, Rouzaud, Sberro-Soussan et al., Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection, Kidney Int
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med
Fda, FDA updates Sotrovimab emergency use authorization
Ganesh, Pawlowski, O'horo, Arndt, Arndt et al., Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19, J Clin Invest
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Heinze, Ploner, Jiricka, logistf: Firth's Bias-Reduced Logistic Regression
Huang, Erin, Mccreary, Bariola, Minnier et al., Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Jarrett, Early Experience With Neutralizing Monoclonal Antibody Therapy For COVID-19
Lynch, Caplan, Furlong, Bateman-House, Helpful Lessons and Cautionary Tales: How Should COVID-19 Drug Development and Access Inform Approaches to Non-Pandemic Diseases?, Am J Bioeth
O'horo, Challener, Speicher, Bosch, Seville et al., Effectiveness of Monoclonal Antibodies in Preventing Severe COVID-19 With Emergence of the Delta Variant, Mayo Clin Proc
Razonable, Pawlowski, O'horo, Arndt, Arndt et al., Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine
Solera, Arbol, Alshahrani, Bahinskaya, Marks et al., Impact of Vaccination and Early Monoclonal Antibody Therapy on COVID-19 Outcomes in Organ Transplant Recipients During the Omicron Wave, Clin Infect Dis
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med
Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants, N Engl J Med
Team, Core, R: a language and environment for statistical computing
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., Investigators Trial. 2021. 'REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Wynia, Beaty, Bennett, Carlson, Davis et al., Real World Evidence of Neutralizing Monoclonal Antibodies for Preventing Hospitalization and Mortality in COVID-19 Outpatients
Yamasoba, Kosugi, Kimura, Fujita, Uriu et al., and Consortium Genotype to Phenotype Japan. 2022. 'Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies, Lancet Infect Dis
Zaqout, Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild to moderate SARS-CoV-2 in Qatar
DOI record:
{
"DOI": "10.1016/j.ijid.2022.10.002",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2022.10.002",
"alternative-id": [
"S1201971222005409"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality for At-risk COVID-19 Outpatients During an Omicron BA.1 and BA.1.1-Predominant Phase"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2022.10.002"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0922-8824",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aggarwal",
"given": "Neil R.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Beaty",
"given": "Laurel E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1483-4236",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bennett",
"given": "Tellen D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0679-6663",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carlson",
"given": "Nichole E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6056-9771",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mayer",
"given": "David A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6764-5984",
"affiliation": [],
"authenticated-orcid": false,
"family": "Molina",
"given": "Kyle C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peers",
"given": "Jennifer",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2436-1367",
"affiliation": [],
"authenticated-orcid": false,
"family": "Russell",
"given": "Seth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3307-069X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wynia",
"given": "Matthew K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T16:12:19Z",
"timestamp": 1665418339000
},
"deposited": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T16:12:19Z",
"timestamp": 1665418339000
},
"indexed": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T16:43:50Z",
"timestamp": 1665420230094
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 2,
"start": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T00:00:00Z",
"timestamp": 1664755200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222005409?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222005409?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971222005409"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality for At-risk COVID-19 Outpatients During an Omicron BA.1 and BA.1.1-Predominant Phase",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
