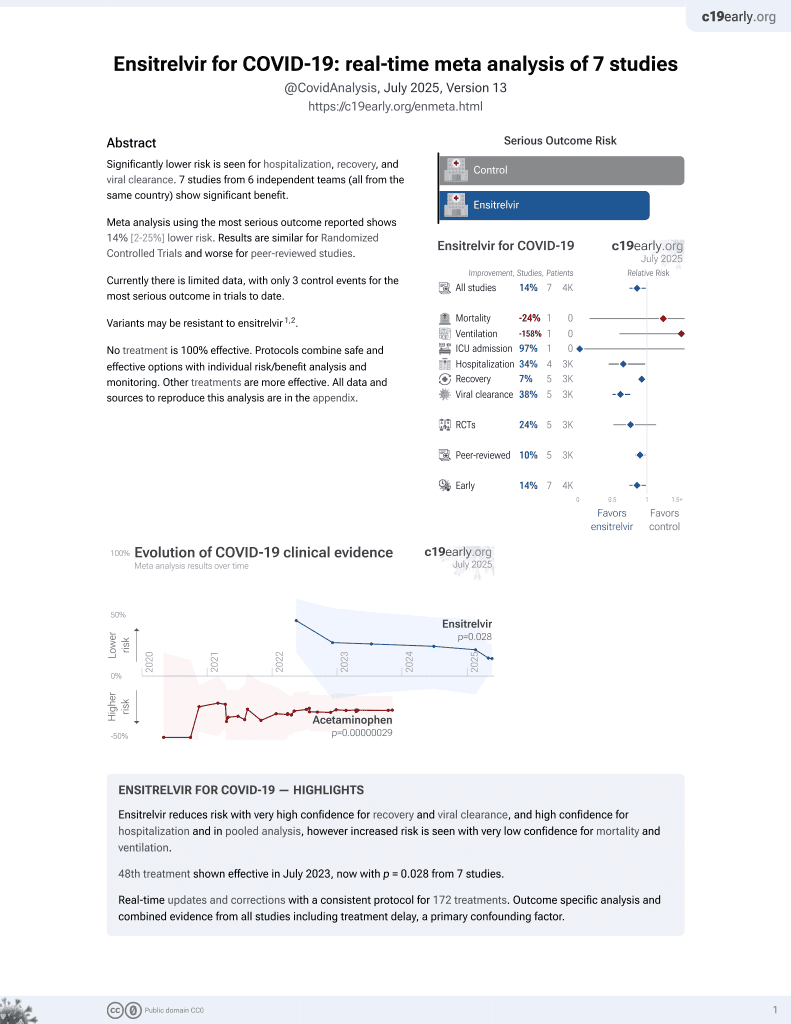
Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac933, jRCT2031210350, Dec 2022
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 428 COVID-19 patients in Japan showing faster viral clearance and improved recovery with ensitrelvir.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
relative improvement in symptom score, 9.2% better, RR 0.91, p = 0.28, treatment mean 5.42 (±3.7) n=116, control mean 4.92 (±3.25) n=111, 250mg, Table 2.
|
|
relative improvement in symptom score, 17.3% better, RR 0.83, p = 0.04, treatment mean 5.95 (±4.02) n=114, control mean 4.92 (±3.25) n=111, 125mg, Table 2.
|
|
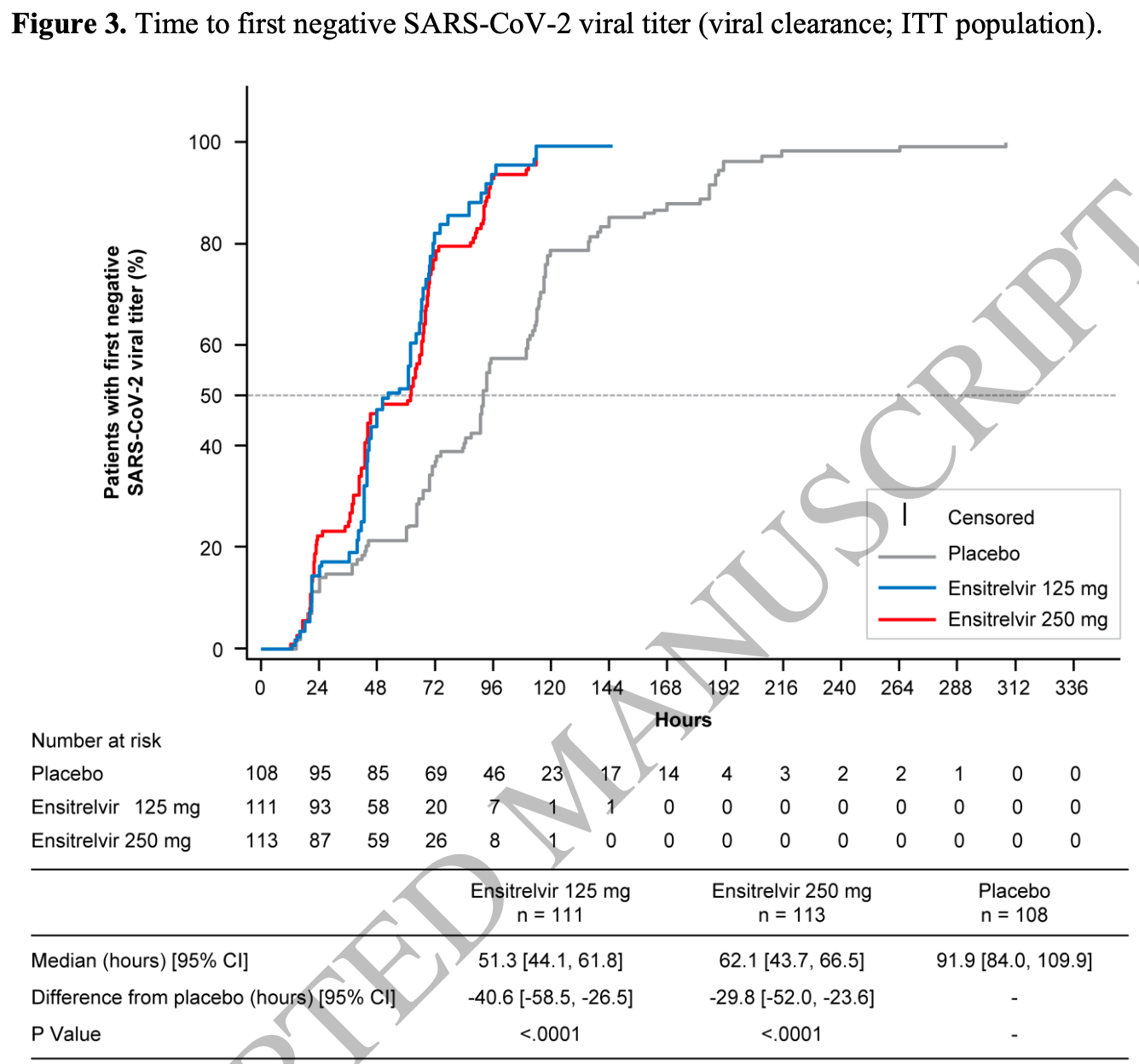
time to viral-, 32.4% lower, relative time 0.68, p < 0.001, treatment 113, control 108, relative time to first negative viral titer, 250mg, Figure 3.
|
|
time to viral-, 44.2% lower, relative time 0.56, p < 0.001, treatment 113, control 108, relative time to first negative viral titer, 125mg, Figure 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mukae et al., 7 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 11 authors, study period 2 January, 2022 - 9 February, 2022, trial jRCT2031210350.
Contact: takeki.uehara@shionogi.co.jp.
Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019 (COVID-19): The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study
Clinical Infectious Diseases, doi:10.1093/cid/ciac933
Background. This phase 2b part of a randomized phase 2/3 study assessed the efficacy and safety of ensitrelvir for mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron epidemic.
References
Ao, Lan, He, SARS-CoV-2 Omicron variant: immune escape and vaccine development, MedComm
Auvigne, Vaux, Strat, Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021 -January 2022: a retrospective, population-based, matched cohort study, eClinicalMedicine
Baden, Sahly, Essink, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med
Bergwerk, Gonen, Lustig, Covid-19 breakthrough infections in vaccinated health care workers, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Blomberg, Mohn, Brokstad, Long COVID in a prospective cohort of home-isolated patients, Nat Med
Fischer, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Food, clinical trials of drugs and biological products for COVID-19 prevention or treatment
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Hay, Kissler, Fauver, Mack, Tai et al., Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant, MedRxiv, doi:10.1101/2022.01.13.22269257v1
Heath, Galiza, Baxter, Safety and efficacy of NVX-CoV2373 Covid-19 vaccine, N Engl J Med
Kumar, Thambiraja, Karuppanan, Subramaniam, Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein, J Med Virol
Levin, Lustig, Cohen, Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months, N Engl J Med
Lopez-Leon, Wegman-Ostrosky, Perelman, More than 50 long-term effects of COVID-19: a systematic review and meta-analysis, Sci Rep
Mautner, Hoyos, Dangel, Berger, Ehrhardt et al., Replication kinetics and infectivity of SARS-CoV-2 variants of concern in common cell culture models, Virol J
Mukae, Yotsuyanagi, Ohmagari, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob Agents Chemother
Nyberg, Ferguson, Nash, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron, Lancet
Pennington, Kompaniyets, Summers, Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19-United States, March-September 2020, Open Forum Infect Dis
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med
Rodebaugh, Frumkin, Reiersen, Acute symptoms of mild to moderate COVID-19 are highly heterogeneous across individuals and over time, Open Forum Infect Dis
Sasaki, Tabata, Kishimoto, Itakura, Kobayashi et al., Oral administration of S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and accelerates recovery from clinical aspects of COVID-19, BioRxiv, doi:10.1101/2022.02.14.480338v1.full
Shimizu, Sonoyama, Fukuhara, Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults, Antimicrob Agents Chemother
Unoh, Uehara, Nakahara, Discovery of S-217622, a non-covalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J Med Chem
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2, Nature
Voysey, Clemens, Madhi, Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials, Lancet
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study, Lancet
DOI record:
{
"DOI": "10.1093/cid/ciac933",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac933",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>This phase 2b part of a randomized phase 2/3 study assessed the efficacy and safety of ensitrelvir for mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron epidemic.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Patients were randomized (1:1:1) to orally receive ensitrelvir fumaric acid 125 mg (375 mg on day 1) or 250 mg (750 mg on day 1) or placebo once daily for 5 days. The co-primary endpoints were the change from baseline in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) titer on day 4 and time-weighted average change from baseline up to 120 hours in the total score of predefined 12 COVID-19 symptoms. Safety was assessed through adverse events.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 341 patients (ensitrelvir 125 mg group, 114; ensitrelvir 250 mg group, 116; and placebo group, 111; male, 53.5%–64.9%; mean age, 35.3–37.3 years) were included in the efficacy analyses. The change from baseline in the SARS-CoV-2 titer on day 4 was significantly greater with both ensitrelvir doses than with placebo (differences from placebo: -0.41 log10 50% tissue-culture infectious dose/mL, P &lt; 0.0001 for both). The total score of the 12 COVID-19 symptoms did not show a significant difference between the ensitrelvir groups and placebo group. The time-weighted average change from baseline up to 120 hours was significantly greater with ensitrelvir versus placebo in several subtotal scores, including acute symptoms and respiratory symptoms. Most adverse events were mild in severity.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Ensitrelvir treatment demonstrated a favorable antiviral efficacy and potential clinical benefit with an acceptable safety profile.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences , Nagasaki , Japan"
}
],
"family": "Mukae",
"given": "Hiroshi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The Institute of Medical Science, The University of Tokyo , Tokyo , Japan"
}
],
"family": "Yotsuyanagi",
"given": "Hiroshi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4622-8970",
"affiliation": [
{
"name": "Disease Control and Prevention Center, National Center for Global Health and Medicine , Tokyo , Japan"
}
],
"authenticated-orcid": false,
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, University of Pittsburgh School of Medicine , Pittsburgh, Pennsylvania , USA"
},
{
"name": "Departments of Microbiology and Infectious Diseases, Fujita Health University School of Medicine , Toyoake , Japan"
}
],
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co., Ltd. , Osaka , Japan"
}
],
"family": "Sakaguchi",
"given": "Hiroki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co., Ltd. , Osaka , Japan"
}
],
"family": "Sonoyama",
"given": "Takuhiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co., Ltd. , Osaka , Japan"
}
],
"family": "Ichihashi",
"given": "Genki",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8847-0071",
"affiliation": [
{
"name": "Pharmaceutical Research Division, Shionogi & Co., Ltd. , Toyonaka , Japan"
}
],
"authenticated-orcid": false,
"family": "Sanaki",
"given": "Takao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Research Division, Shionogi & Co., Ltd. , Toyonaka , Japan"
}
],
"family": "Baba",
"given": "Keiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co., Ltd. , Osaka , Japan"
}
],
"family": "Tsuge",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co., Ltd. , Osaka , Japan"
}
],
"family": "Uehara",
"given": "Takeki",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T07:28:23Z",
"timestamp": 1670398103000
},
"deposited": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T16:31:04Z",
"timestamp": 1670430664000
},
"indexed": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T17:14:13Z",
"timestamp": 1670433253037
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
7
]
],
"date-time": "2022-12-07T00:00:00Z",
"timestamp": 1670371200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac933/47684795/ciac933.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac933/47684795/ciac933.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
12,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
7
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac933/6881001"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study",
"type": "journal-article"
}