Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy
et al., medRxiv, doi:10.1101/2021.09.03.21263105, NCT04518410, Sep 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
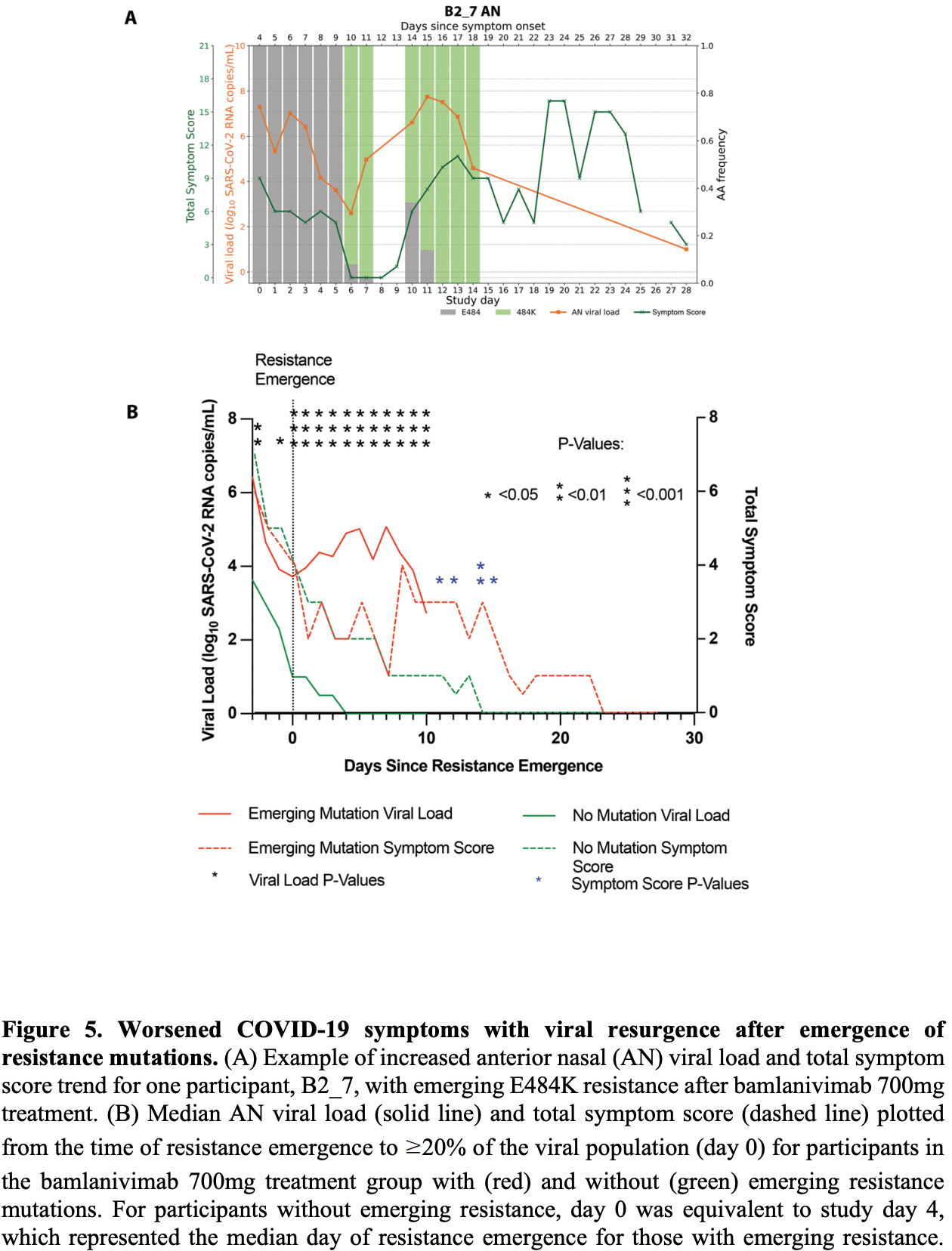
Analysis of ACTIV-2/A5401, showing the potential for rapid emergence of resistance during monoclonal antibody treatment, resulting in prolonged high level viral loads and clinical worsening. Treatment-emergent resistance mutations were more common after bamlanivimab 700mg (7% of 111 vs 0% of 112 participants, p=0.003).
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
Choudhary et al., 15 Sep 2021, preprint, 28 authors, trial NCT04518410 (history).
Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy
doi:10.1101/2021.09.03.21263105
All rights reserved. No reuse allowed without permission. preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
Author contributions MCC, KC, RC, DMS, JZL conceptualized and performed the study, MCC, RD, JPF, JR, CC performed resistance analysis experiment; RMR, RK, ASP performed mathematical modeling; JD, AG performed viral load analysis; UP, SS performed serological analysis; CM, MH, JR performed statistical analysis.
References
Berg, Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity, J Clin Virol, doi:10.1016/j.jcv.2021.104945
Campbell, Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021, Euro Surveill, doi:10.2807/1560-7917.ES.2021.26.24.2100509
Chen, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Chew, Conference on Retroviruses and Opportunistic Infections (Virtual
Choi, Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host, N Engl J Med, doi:10.1056/NEJMc2031364
Chou, Guentzel, Michels, Miner, Drew, Frequency of UL97 phosphotransferase mutations related to ganciclovir resistance in clinical cytomegalovirus isolates, J Infect Dis, doi:10.1093/infdis/172.1.239
Choudhary, Crain, Qiu, Hanage, Li, SARS-CoV-2 Sequence Characteristics of COVID-19 Persistence and Reinfection, Clin Infect Dis, doi:10.1093/cid/ciab380
Clavel, Hance, HIV drug resistance, N Engl J Med, doi:10.1056/NEJMra025195
Collier, Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies, Nature, doi:10.1038/s41586-021-03412-7
Degli-Angeli, Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance, J Clin Virol, doi:10.1016/j.jcv.2020.104474
Fajnzylber, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Gottlieb, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.0202
Gupta, Will SARS-CoV-2 variants of concern affect the promise of vaccines?, Nat Rev Immunol, doi:10.1038/s41577-021-00556-5
He, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med, doi:10.1038/s41591-020-0869-5
Ko, High-throughput, single-copy sequencing reveals SARS-CoV-2 spike variants coincident with mounting humoral immunity during acute COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1009431
Larder, Kemp, Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT), Science, doi:10.1126/science.2479983
Lee, Performance comparison of next generation sequencing analysis pipelines for HIV-1 drug resistance testing, Sci Rep, doi:10.1038/s41598-020-58544-z
Luan, Wang, Huynh, Enhanced binding of the N501Y-mutated SARS-CoV-2 spike protein to the human ACE2 receptor: insights from molecular dynamics simulations, FEBS Lett, doi:10.1002/1873-3468.14076
Mccallum, SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern, Science, doi:10.1126/science.abi7994
Mccarthy, Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape, Science, doi:10.1126/science.abf6950
Moore, Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level, Science
Pelegrin, Naranjo-Gomez, Piechaczyk, Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents?, Trends Microbiol, doi:10.1016/j.tim.2015.07.005
Plant, Manukyan, Sanchez, Laassri, Ye, Immune Pressure on Polymorphous Influenza B Populations Results in Diverse Hemagglutinin Escape Mutants and Lineage Switching, Vaccines, doi:10.3390/vaccines8010125
Sheet, Health, Providers, EMERGENCY USE AUTHORIZATION (EUA) OF BAMLANIVIMAB AND ETESEVIMAB
Sheet, Health, Providers, EMERGENCY USE AUTHORIZATION (EUA) OF CASIRIVIMAB AND IMDEVIMAB
Starr, Greaney, Dingens, Bloom, Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016, Cell Rep Med, doi:10.1016/j.xcrm.2021.100255
Starr, Prospective mapping of viral mutations that escape antibodies used to treat COVID-19, Science, doi:10.1126/science.abf9302
Takashita, Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2017-2018, Antiviral Res, doi:10.1016/j.antiviral.2020.104718
Truong, Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: A consecutive case series, EBioMedicine, doi:10.1016/j.ebiom.2021.103355
Weinreich, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wolfel, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Zhou, Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD, Cell Rep, doi:10.1016/j.celrep.2021.108699
DOI record:
{
"DOI": "10.1101/2021.09.03.21263105",
"URL": "http://dx.doi.org/10.1101/2021.09.03.21263105",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:p>Resistance mutations to monoclonal antibody (mAb) therapy has been reported, but in the non-immunosuppressed population, it is unclear if <jats:italic>in vivo</jats:italic> emergence of SARS-CoV-2 resistance mutations alters either viral replication dynamics or therapeutic efficacy. In ACTIV-2/A5401, non-hospitalized participants with symptomatic SARS-CoV-2 infection were randomized to bamlanivimab (700mg or 7000mg) or placebo. Treatment-emergent resistance mutations were significantly more likely detected after bamlanivimab 700mg treatment than placebo (7% of 111 vs 0% of 112 participants, P=0.003). There were no treatment-emergent resistance mutations among the 48 participants who received bamlanivimab 7000mg. Participants with emerging mAb resistant virus had significantly higher pre-treatment nasopharyngeal and anterior nasal viral load. Intensive respiratory tract viral sampling revealed the dynamic nature of SARS-CoV-2 evolution, with evidence of rapid and sustained viral rebound after emergence of resistance mutations, and worsened symptom severity. Participants with emerging bamlanivimab resistance often accumulated additional polymorphisms found in current variants of concern/interest and associated with immune escape. These results highlight the potential for rapid emergence of resistance during mAb monotherapy treatment, resulting in prolonged high level respiratory tract viral loads and clinical worsening. Careful virologic assessment should be prioritized during the development and clinical implementation of antiviral treatments for COVID-19.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
9,
15
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3192-7840",
"affiliation": [],
"authenticated-orcid": false,
"family": "Choudhary",
"given": "Manish C.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Chew",
"given": "Kara W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deo",
"given": "Rinki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flynn",
"given": "James P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Regan",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crain",
"given": "Charles R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moser",
"given": "Carlee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ritz",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ribeiro",
"given": "Ruy M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ke",
"given": "Ruian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dragavon",
"given": "Joan A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Javan",
"given": "Arzhang C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nirula",
"given": "Ajay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klekotka",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greninger",
"given": "Alexander L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fletcher",
"given": "Courtney V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daar",
"given": "Eric S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wohl",
"given": "David A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eron",
"given": "Joseph J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Currier",
"given": "Judith S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parikh",
"given": "Urvi M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sieg",
"given": "Scott F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perelson",
"given": "Alan S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coombs",
"given": "Robert W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Davey M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jonathan Z.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the ACTIV-2/A5401 Study Team",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
15
]
],
"date-time": "2021-09-15T11:45:12Z",
"timestamp": 1631706312000
},
"deposited": {
"date-parts": [
[
2021,
9,
16
]
],
"date-time": "2021-09-16T17:10:35Z",
"timestamp": 1631812235000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
1,
3
]
],
"date-time": "2022-01-03T17:26:58Z",
"timestamp": 1641230818693
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
9,
15
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.09.03.21263105",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
9,
15
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
9,
15
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1126/science.1069660",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.1"
},
{
"DOI": "10.3390/vaccines8010125",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.2"
},
{
"DOI": "10.1126/science.2479983",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.3"
},
{
"DOI": "10.1093/infdis/172.1.239",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.4"
},
{
"DOI": "10.1016/j.antiviral.2020.104718",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.5"
},
{
"DOI": "10.1056/NEJM2ra025195",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.6"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.7"
},
{
"key": "2021091610100424000_2021.09.03.21263105v1.8",
"unstructured": "FACT SHEET FOR HEALTH CARE PROVIDERS: EMERGENCY USE AUTHORIZATION (EUA) OF CASIRIVIMAB AND IMDEVIMAB, < https://www.fda.gov/media/143892/download> (Accessed July 14, 2021)."
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.9"
},
{
"DOI": "10.1016/j.xcrm.2021.100255",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.10"
},
{
"DOI": "10.1126/science.abf9302",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.11"
},
{
"DOI": "10.1126/science.abi7994",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.12"
},
{
"key": "2021091610100424000_2021.09.03.21263105v1.13",
"unstructured": "FACT SHEET FOR HEALTH CARE PROVIDERS: EMERGENCY USE AUTHORIZATION (EUA) OF BAMLANIVIMAB AND ETESEVIMAB, < https://www.fda.gov/media/145802/download> (Accessed July 14, 2021)."
},
{
"DOI": "10.1016/j.ebiom.2021.103355",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.14"
},
{
"DOI": "10.1056/NEJMc2031364",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.15"
},
{
"DOI": "10.1126/science.abf6950",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.16"
},
{
"DOI": "10.1002/1873-3468.14076",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.17"
},
{
"DOI": "10.1038/s41577-021-00556-5",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.18"
},
{
"key": "2021091610100424000_2021.09.03.21263105v1.19",
"unstructured": "Chew, K. W. et al. in Conference on Retroviruses and Opportunistic Infections (Virtual, March 6-10, 2021 (Abstract 396))."
},
{
"DOI": "10.1371/journal.ppat.1009431",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.20"
},
{
"DOI": "10.1093/cid/ciab380",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.21"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.22"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.23"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.24"
},
{
"DOI": "10.1016/j.tim.2015.07.005",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.25"
},
{
"DOI": "10.1016/j.celrep.2021.108699",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.26"
},
{
"DOI": "10.1038/s41586-021-03412-7",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.27"
},
{
"DOI": "10.2807/1560-7917.ES.2021.26.24.2100509",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.28"
},
{
"article-title": "An EUA for sotrovimab for treatment of COVID-19",
"first-page": "97",
"journal-title": "Med Lett Drugs Ther",
"key": "2021091610100424000_2021.09.03.21263105v1.29",
"volume": "63",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2020.104474",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.30"
},
{
"DOI": "10.1016/j.jcv.2021.104945",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.31"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.32"
},
{
"DOI": "10.1038/s41598-020-58544-z",
"doi-asserted-by": "publisher",
"key": "2021091610100424000_2021.09.03.21263105v1.33"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy"
],
"type": "posted-content"
}
