SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19
et al., NEJM, doi:10.1056/NEJMoa2029849, NCT04427501, Oct 2020
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
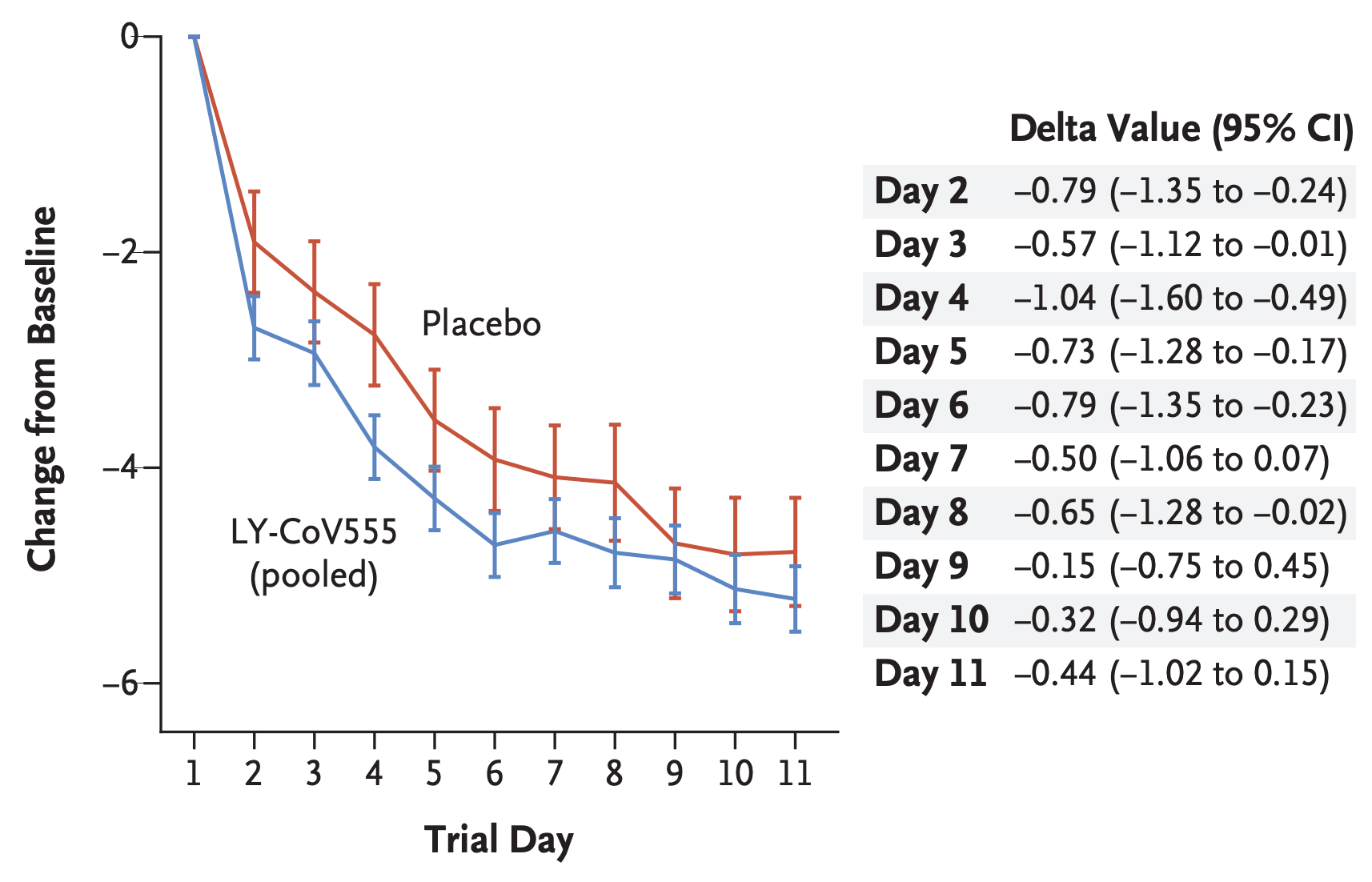
Interim analysis of the BLAZE-1 phase 2 trial of outpatients showing lower hospitalization or ER visits (1.6% versus 6.3%), and improvements in symptoms and viral load compared to placebo.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 74.3% lower, RR 0.26, p = 0.02, treatment 5 of 309 (1.6%), control 9 of 143 (6.3%), NNT 21.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Chen et al., 28 Oct 2020, Randomized Controlled Trial, USA, peer-reviewed, 12 authors, study period 17 June, 2020 - 5 September, 2020, average treatment delay 4.0 days, trial NCT04427501 (history).
SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2029849
BACKGROUND Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (Covid-19), which is most frequently mild yet can be severe and lifethreatening. Virus-neutralizing monoclonal antibodies are predicted to reduce viral load, ameliorate symptoms, and prevent hospitalization.
METHODS In this ongoing phase 2 trial involving outpatients with recently diagnosed mild or moderate Covid-19, we randomly assigned 452 patients to receive a single intravenous infusion of neutralizing antibody LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo and evaluated the quantitative virologic end points and clinical outcomes. The primary outcome was the change from baseline in the viral load at day 11. The results of a preplanned interim analysis as of September 5, 2020, are reported here.
RESULTS At the time of the interim analysis, the observed mean decrease from baseline in the log viral load for the entire population was −3.81, for an elimination of more than 99.97% of viral RNA. For patients who received the 2800-mg dose of LY-CoV555, the difference from placebo in the decrease from baseline was −0.53 (95% confidence interval [CI], −0.98 to −0.08; P = 0.02), for a viral load that was lower by a factor of 3.4. Smaller differences from placebo in the change from baseline were observed among the patients who received the 700-mg dose (−0.20; 95% CI, −0.66 to 0.25; P = 0.38) or the 7000-mg dose (0.09; 95% CI, −0.37 to 0.55; P = 0.70). On days 2 to 6, the patients who received LY-CoV555 had a slightly lower severity of symptoms than those who received placebo. The percentage of patients who had a Covid-19-related hospitalization or visit to an emergency department was 1.6% in the LY-CoV555 group and 6.3% in the placebo group.
CONCLUSIONS In this interim analysis of a phase 2 trial, one of three doses of neutralizing antibody LY-CoV555 appeared to accelerate the natural decline in viral load over time, whereas the other doses had not by day 11. (Funded by Eli Lilly; BLAZE-1 ClinicalTrials.gov number, NCT04427501.
References
Baum, Ajithdoss, Copin, REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters, Science
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Bronte, Ugel, Tinazzi, Baricitinib restrains the immune dysregulation in severe COVID-19 patients, J Clin Invest
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19
Chen, Qi, Liu, Clinical progression of patients with COVID-19 in Shanghai, China, J Infect
Dastan, Saffaei, Haseli, Promising effects of tocilizumab in COV-ID-19: a non-controlled, prospective clinical trial, Int Immunopharmacol
Farooqi, Dhawan, Morgan, Dinh, Nedd et al., Treatment of severe COVID-19 with tocilizumab mitigates cytokine storm and averts mechanical ventilation during acute respiratory distress: a case report and literature review, Trop Med Infect Dis
Garg, Kim, Whitaker, Hospitalization rates and characteristics of patients hospitalized with laboratoryconfirmed coronavirus disease 2019 -COVID-NET, 14 states, MMWR Morb Mortal Wkly Rep
Goldman, Lye, Hui, Remdesivir for 5 or 10 days in patients with severe Covid-19, N Engl J Med
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Jehi, Ji, Milinovich, Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19, PLoS One
Jones, Brown-Augsburger, Corbett, LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection, doi:10.1101/2020.09.30.318972v3
Joyner, Senefeld, Klassen, Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, doi:10.1101/2020.08.12.20169359v1
Kim, Garg, 'halloran, Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET), Clin Infect Dis
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Liu, Yan, Wan, Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis
Sims, Krishnan, Chang, Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19, J Allergy Clin Immunol
Spinner, Gottlieb, Criner, Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA
Stebbing, Krishnan, De Bono, Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients, EMBO Mol Med
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COV-ID-19: a meta-analysis, JAMA
Tabata, Imai, Kawano, Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis, Lancet Infect Dis
The, Group, Dexamethasone in hospitalized patients with Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1056/nejmoa2029849",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2029849",
"alternative-id": [
"10.1056/NEJMoa2029849"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5330-1718",
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"authenticated-orcid": false,
"family": "Chen",
"given": "Peter",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Nirula",
"given": "Ajay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Heller",
"given": "Barry",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8376-8709",
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"authenticated-orcid": false,
"family": "Gottlieb",
"given": "Robert L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Boscia",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Morris",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Huhn",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Cardona",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Mocherla",
"given": "Bharat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Stosor",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Shawa",
"given": "Imad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Adams",
"given": "Andrew C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Van Naarden",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Custer",
"given": "Kenneth L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Shen",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Durante",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Oakley",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Schade",
"given": "Andrew E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Sabo",
"given": "Janelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Patel",
"given": "Dipak R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Klekotka",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — both in California; Eli Lilly, Indianapolis (A.N., A.C.A., J.V.N., K.L.C., L.S., M.D., G.O., A.E.S., J.S., D.R.P., P.K., D.M.S.), and Franciscan Health, Greenwood (I.S.) — both in Indiana; Baylor University Medical Center and Baylor Scott and White Research Institute, Dallas (R.L.G.); Vitalink Research, Union, SC (J.B.); Imperial Health, Lake..."
}
],
"family": "Skovronsky",
"given": "Daniel M.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
28
]
],
"date-time": "2020-10-28T21:01:15Z",
"timestamp": 1603918875000
},
"deposited": {
"date-parts": [
[
2023,
5,
5
]
],
"date-time": "2023-05-05T17:51:29Z",
"timestamp": 1683309089000
},
"funder": [
{
"DOI": "10.13039/100004312",
"doi-asserted-by": "publisher",
"name": "Eli Lilly and Company"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T17:58:16Z",
"timestamp": 1715709496515
},
"is-referenced-by-count": 1055,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
1,
21
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2021,
1,
21
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
21
]
],
"date-time": "2021-01-21T00:00:00Z",
"timestamp": 1611187200000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2029849",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "229-237",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
1,
21
]
]
},
"published-print": {
"date-parts": [
[
2021,
1,
21
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30482-5",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1371/journal.pone.0237419",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1056/NEJMoa2019014",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1056/NEJMoa2015301",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1001/jama.2020.16349",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1016/j.jaci.2020.08.031",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1172/JCI141772",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.15252/emmm.202012697",
"doi-asserted-by": "publisher",
"key": "r10"
},
{
"DOI": "10.1016/j.intimp.2020.106869",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.3390/tropicalmed5030112",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"author": "Sterne JAC",
"first-page": "1",
"journal-title": "JAMA",
"key": "r14",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"doi-asserted-by": "publisher",
"key": "r16"
},
{
"DOI": "10.1126/science.abe2402",
"doi-asserted-by": "publisher",
"key": "r17"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "r18"
},
{
"DOI": "10.1016/j.jinf.2020.03.004",
"doi-asserted-by": "publisher",
"key": "r20"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"doi-asserted-by": "publisher",
"key": "r21"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"doi-asserted-by": "publisher",
"key": "r22"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "r23"
},
{
"DOI": "10.1093/cid/ciaa1012",
"doi-asserted-by": "publisher",
"key": "r24"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2029849"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19",
"type": "journal-article",
"volume": "384"
}
