Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): a randomised, parallel-arm, open-label, phase 4 trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(23)00376-4, TACTIC-R, NCT04390464, Dec 2023
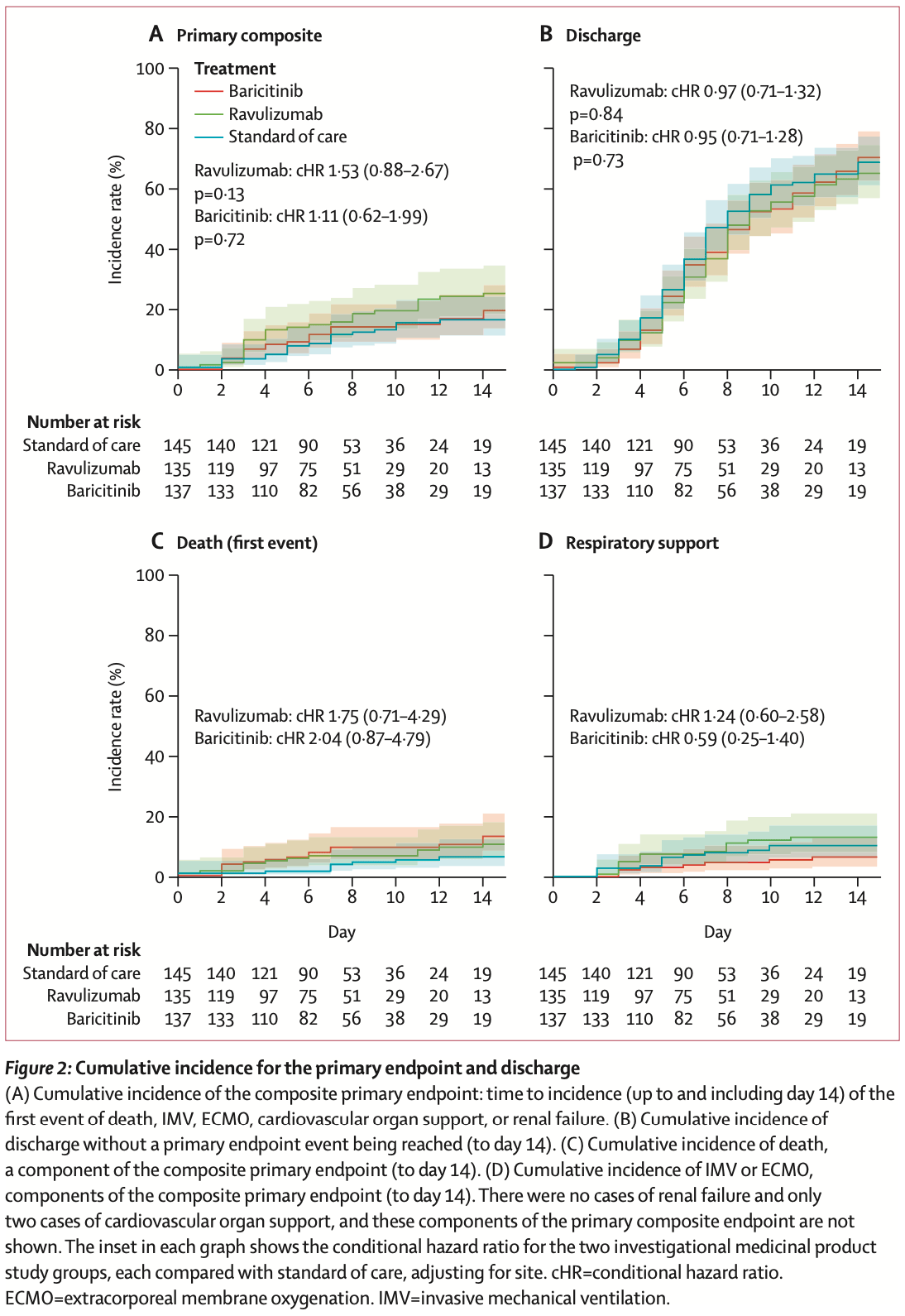
RCT 417 hospitalized COVID-19 patients in the UK showing no significant difference in a composite primary endpoint (time to death, invasive mechanical ventilation, extracorporeal membrane oxygenation, cardiovascular organ support, or renal failure) with baricitinib or ravulizumab. The trial was stopped early due to futility. There were no significant differences in secondary outcomes including disease severity, days to discharge, mortality, or adverse events.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 1.0% lower, HR 0.99, p = 0.98, treatment 135, control 145, day 28.
|
|
risk of death, 75.0% higher, HR 1.75, p = 0.22, treatment 11 of 135 (8.1%), control 7 of 145 (4.8%), adjusted per study, multivariable, Cox proportional hazards, day 14.
|
|
death, mechanical ventilation, ECMO, cardiovascular organ support, renal failure, 53.0% higher, HR 1.53, p = 0.13, treatment 135, control 145, adjusted per study, multivariable, Cox proportional hazards, day 14.
|
|
risk of oxygen therapy, 24.0% higher, HR 1.24, p = 0.57, treatment 135, control 145, adjusted per study, multivariable, Cox proportional hazards, day 14.
|
|
risk of no hospital discharge, 3.1% higher, HR 1.03, p = 0.86, treatment 135, control 145, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hall et al., 31 Dec 2023, Randomized Controlled Trial, United Kingdom, peer-reviewed, 170 authors, study period 8 May, 2020 - 7 May, 2021, trial NCT04390464 (history) (TACTIC-R).
Contact: fch22@medschl.cam.ac.uk.
Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): a randomised, parallel-arm, open-label, phase 4 trial
Background From early in the COVID-19 pandemic, evidence suggested a role for cytokine dysregulation and complement activation in severe disease. In the TACTIC-R trial, we evaluated the efficacy and safety of baricitinib, an inhibitor of Janus kinase 1 (JAK1) and JAK2, and ravulizumab, a monoclonal inhibitor of complement C5 activation, as an adjunct to standard of care for the treatment of adult patients hospitalised with COVID-19.
Methods TACTIC-R was a phase 4, randomised, parallel-arm, open-label platform trial that was undertaken in the UK with urgent public health designation to assess the potential of repurposing immunosuppressants for the treatment of severe COVID-19, stratified by a risk score. Adult participants (aged ≥18 years) were enrolled from 22 hospitals across the UK. Patients with a risk score indicating a 40% risk of admission to an intensive care unit or death were randomly assigned 1:1:1 to standard of care alone, standard of care with baricitinib, or standard of care with ravulizumab. The composite primary outcome was the time from randomisation to incidence (up to and including day 14) of the first event of death, invasive mechanical ventilation, extracorporeal membrane oxygenation, cardiovascular organ support, or renal failure. The primary interim analysis was triggered when 125 patient datasets were available up to day 14 in each study group and we included in the analysis all participants who were randomly assigned. The trial was registered on ClinicalTrials.gov (NCT04390464). Findings Between May 8, 2020, and May 7, 2021, 417 participants were recruited and randomly assigned to standard of care alone (145 patients), baricitinib (137 patients), or ravulizumab (135 patients). Only 54 (39%) of 137 patients in the baricitinib group received the maximum 14-day course, whereas 132 (98%) of 135 patients in the ravulizumab group received the intended dose. The trial was stopped after the primary interim analysis on grounds of futility. The estimated hazard ratio (HR) for reaching the composite primary endpoint was 1•11 (95% CI 0•62-1•99) for patients on baricitinib compared with standard of care alone, and 1•53 (0•88-2•67) for ravulizumab compared with standard of care alone. 45 serious adverse events (21 deaths) were reported in the standard-of-care group, 57 (24 deaths) in the baricitinib group, and 60 (18 deaths) in the ravulizumab group. Interpretation Neither baricitinib nor ravulizumab, as administered in this study, was effective in reducing disease severity in patients selected for severe COVID-19. Safety was similar between treatments and standard of care. The short period of dosing with baricitinib might explain the discrepancy between our findings and those of other trials. The therapeutic potential of targeting complement C5 activation product C5a, rather than the cleavage of C5, warrants further evaluation.
References
Ackermann, Verleden, Kuehnel, Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19, N Engl J Med
Altman, Practical statistics for medical research
Annane, Pittock, Kulkarni, Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: a phases 3, multicentre, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(23)00082-6
Bergamaschi, Mescia, Turner, Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease, Immunity
Carvelli, Meziani, Dellamonica, Avdoralimab (anti-C5aR1 mAb) versus placebo in patients with severe COVID-19: results from a randomized controlled trial (for COVID elimination [FORCE]), Crit Care Med
Coutinho, Chapman, The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights, Mol Cell Endocrinol
De Leeuw, Van Damme, Declercq, Efficacy and safety of the investigational complement C5 inhibitor zilucoplan in patients hospitalized with COVID-19: an open-label randomized controlled trial, Respir Res
Galloway, Norton, Barker, A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study, J Infect
Gralinski, Sheahan, Morrison, Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis, MBio
Grambsch, Therneau, Proportional hazards tests and diagnostics based on weighted residuals, Biometrika
Gudu, Stober, Cope, Baricitinib set to join the COVID-19 therapeutic arsenal?, Rheumatology
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Hu, Sun, Dai, Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis, J Clinical Virol
Ip, Chan, Law, Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection, J Infect Dis
Jiang, Zhao, Song, Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV, Emerg Microbes Infect
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med
Kulasekararaj, Hill, Langemeijer, One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab, Eur J Haematol
Kulkarni, Fisk, Kostapanos, Repurposed immunomodulatory drugs for COVID-19 in pre-ICu patients-mulTi-Arm therapeutic study in pre-ICu patients admitted with COVID-19-repurposed drugs (TACTIC-R): a structured summary of a study protocol for a randomised controlled trial, Trials
Maccio, Zinkernagel, Shambat, SARS-CoV-2 leads to a small vessel endotheliitis in the heart, EBioMedicine
Magro, Mulvey, Kubiak, Severe COVID-19: a multifaceted viral vasculopathy syndrome, Ann Diagn Pathol
Mansournia, Nazemipour, Etminan, A practical guide to handling competing events in etiologic time-to-event studies, Glob Epidemiol
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med
Messner, Demichev, Wendisch, Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection, Cell Syst
Mosleh, Chen, Pfau, Vashist, Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes, J Clin Med
Ruggenenti, Marco, Cortinovis, Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: retrospective cohort study, PLoS One
Varga, Flammer, Steiger, Endothelial cell infection and endotheliitis in COVID-19, Lancet
Vlaar, Witzenrath, Van Paassen, Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial, Lancet Respir Med
DOI record:
{
"DOI": "10.1016/s2213-2600(23)00376-4",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(23)00376-4",
"alternative-id": [
"S2213260023003764"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): a randomised, parallel-arm, open-label, phase 4 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(23)00376-4"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(23)00423-X"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Hall",
"given": "Frances C",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cheriyan",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cope",
"given": "Andrew P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galloway",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bond",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norton",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banham-Hall",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayes",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kostapanos",
"given": "Michalis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nodale",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petchey",
"given": "William G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheeran",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Underwood",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jayne",
"given": "David R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hall",
"given": "Frances C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheriyan",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cope",
"given": "Andrew P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galloway",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bond",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norton",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banham-Hall",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayes",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kostapanos",
"given": "Michalis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nodale",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petchey",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheeran",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Underwood",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jayne",
"given": "David R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galloway",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagra",
"given": "Deepak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norton",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bird",
"given": "Georgina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Underwood",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Rhys John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forde",
"given": "Donall",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nye",
"given": "Clemency",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balan",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bird",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Britten",
"given": "Vianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Broad",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Teriann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frayling",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gray",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haynes",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliver",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahilly",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Tanwir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayliss",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Byrne",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernan-Sancho",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kasanicki",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stockley",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Templin",
"given": "Heike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kostapanos",
"given": "Michalis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheriyan",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banham-Hall",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fisk",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graggaber",
"given": "Johann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gray",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gudu",
"given": "Tania",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kulkarni",
"given": "Spoorthy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ni Lu",
"given": "Ing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masters",
"given": "Peta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mir",
"given": "Fraz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stober",
"given": "Carmel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abercrombie",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bermperi",
"given": "Areti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burns",
"given": "Stella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canna",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Domingo",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hodges",
"given": "Kathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jose",
"given": "Sherly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kourampa",
"given": "Evgenia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meadows",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendoza",
"given": "Vivien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mushapaizdi",
"given": "Thelma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nacorda",
"given": "Aileen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pasquale",
"given": "Ciro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Read",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rowlands",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruffulo",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soave",
"given": "Carlotta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Titti",
"given": "Lissamma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tordesillas",
"given": "Hugo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wright",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayes",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scott",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Varun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cathcart",
"given": "Susanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rimmer",
"given": "Dominic",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Semple",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheeran",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phiri",
"given": "Laurence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plumbe",
"given": "Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petchey",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhagat",
"given": "Shweta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moody",
"given": "Anne Margaret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kellett",
"given": "Jo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bukhari",
"given": "Marwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burns",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crayton",
"given": "Susanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fielding",
"given": "Andra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simpson",
"given": "Kerry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thatcher",
"given": "Hilary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makkuni",
"given": "Damodar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harrison",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jain",
"given": "Thrusha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patrick",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pratt",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheerin",
"given": "Neil S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kavanagh",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barr",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baldwin",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Judd",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McAlinden",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCormack",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stephenson",
"given": "Elaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Sunil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cross",
"given": "Elizabeth L A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bracewell",
"given": "Kirsty",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Musiol",
"given": "Monika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seal",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cope",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koduri",
"given": "Gouri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercioniu",
"given": "Mihaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kunhunny",
"given": "Swapna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pollard",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coakley",
"given": "Gerald",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Sunil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holloway",
"given": "Amelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilgrim",
"given": "Samia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Treus",
"given": "Estefania",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boehmer",
"given": "Gabriele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beranova",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ionita",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deery",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hazelton",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knight",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Price",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tilbey",
"given": "Sorrell",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bradbury",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Willis",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drury",
"given": "Kay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Putensen",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmidtmann",
"given": "Anja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galloway",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dimitriadis",
"given": "Georgios K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gogoi",
"given": "Sukanya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vidler",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajasekaran",
"given": "Arvind",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orme",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devenport",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nisar",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindergard",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uriel",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hey",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamb",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruce",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flaherty",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parfrey",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dorey",
"given": "Kane",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
14
]
],
"date-time": "2023-11-14T23:31:17Z",
"timestamp": 1700004677000
},
"deposited": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T06:37:36Z",
"timestamp": 1715063856000
},
"funder": [
{
"DOI": "10.13039/100004312",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100004312",
"id-type": "DOI"
}
],
"name": "Eli Lilly and Company"
},
{
"DOI": "10.13039/100006396",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100006396",
"id-type": "DOI"
}
],
"name": "Alexion Pharmaceuticals Inc Lexington"
},
{
"DOI": "10.13039/501100000265",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000265",
"id-type": "DOI"
}
],
"name": "UKRI Medical Research Council"
},
{
"DOI": "10.13039/501100018956",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100018956",
"id-type": "DOI"
}
],
"name": "NIHR Cambridge Biomedical Research Centre"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
27
]
],
"date-time": "2024-08-27T19:05:58Z",
"timestamp": 1724785558123
},
"is-referenced-by-count": 8,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
10
]
],
"date-time": "2023-10-10T00:00:00Z",
"timestamp": 1696896000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260023003764?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260023003764?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1064-1074",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.jcv.2020.104371",
"article-title": "Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis",
"author": "Hu",
"doi-asserted-by": "crossref",
"journal-title": "J Clinical Virol",
"key": "10.1016/S2213-2600(23)00376-4_bib1",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19",
"author": "Ackermann",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(23)00376-4_bib2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19",
"author": "Varga",
"doi-asserted-by": "crossref",
"first-page": "1417",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(23)00376-4_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/rheumatology/keab061",
"article-title": "Baricitinib set to join the COVID-19 therapeutic arsenal?",
"author": "Gudu",
"doi-asserted-by": "crossref",
"first-page": "1585",
"journal-title": "Rheumatology (Oxford)",
"key": "10.1016/S2213-2600(23)00376-4_bib4",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1128/mBio.01753-18",
"article-title": "Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis",
"author": "Gralinski",
"doi-asserted-by": "crossref",
"first-page": "e01753",
"journal-title": "MBio",
"key": "10.1016/S2213-2600(23)00376-4_bib5",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1086/429631",
"article-title": "Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection",
"author": "Ip",
"doi-asserted-by": "crossref",
"first-page": "1697",
"journal-title": "J Infect Dis",
"key": "10.1016/S2213-2600(23)00376-4_bib6",
"volume": "191",
"year": "2005"
},
{
"DOI": "10.1038/s41426-018-0063-8",
"article-title": "Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Emerg Microbes Infect",
"key": "10.1016/S2213-2600(23)00376-4_bib7",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1016/j.anndiagpath.2020.151645",
"article-title": "Severe COVID-19: a multifaceted viral vasculopathy syndrome",
"author": "Magro",
"doi-asserted-by": "crossref",
"journal-title": "Ann Diagn Pathol",
"key": "10.1016/S2213-2600(23)00376-4_bib8",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1016/j.cels.2020.05.012",
"article-title": "Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection",
"author": "Messner",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Cell Syst",
"key": "10.1016/S2213-2600(23)00376-4_bib9",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.103182",
"article-title": "SARS-CoV-2 leads to a small vessel endotheliitis in the heart",
"author": "Maccio",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/S2213-2600(23)00376-4_bib10",
"volume": "63",
"year": "2021"
},
{
"DOI": "10.3390/jcm9061862",
"article-title": "Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes",
"author": "Mosleh",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "10.1016/S2213-2600(23)00376-4_bib11",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/ejh.13564",
"article-title": "One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab",
"author": "Kulasekararaj",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Eur J Haematol",
"key": "10.1016/S2213-2600(23)00376-4_bib12",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04535-4",
"article-title": "Repurposed immunomodulatory drugs for COVID-19 in pre-ICu patients—mulTi-Arm therapeutic study in pre-ICu patients admitted with COVID-19—repurposed drugs (TACTIC-R): a structured summary of a study protocol for a randomised controlled trial",
"author": "Kulkarni",
"doi-asserted-by": "crossref",
"first-page": "626",
"journal-title": "Trials",
"key": "10.1016/S2213-2600(23)00376-4_bib13",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.05.064",
"article-title": "A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study",
"author": "Galloway",
"doi-asserted-by": "crossref",
"first-page": "282",
"journal-title": "J Infect",
"key": "10.1016/S2213-2600(23)00376-4_bib14",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1093/biomet/81.3.515",
"article-title": "Proportional hazards tests and diagnostics based on weighted residuals",
"author": "Grambsch",
"doi-asserted-by": "crossref",
"first-page": "515",
"journal-title": "Biometrika",
"key": "10.1016/S2213-2600(23)00376-4_bib15",
"volume": "81",
"year": "1994"
},
{
"article-title": "A practical guide to handling competing events in etiologic time-to-event studies",
"author": "Mansournia",
"journal-title": "Glob Epidemiol",
"key": "10.1016/S2213-2600(23)00376-4_bib16",
"volume": "4",
"year": "2022"
},
{
"author": "Altman",
"key": "10.1016/S2213-2600(23)00376-4_bib17",
"series-title": "Practical statistics for medical research",
"year": "1991"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(23)00376-4_bib18",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)01109-6",
"article-title": "Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(23)00376-4_bib20",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(23)00376-4_bib21",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(23)00376-4_bib22",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0261113",
"article-title": "Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: retrospective cohort study",
"author": "Ruggenenti",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(23)00376-4_bib23",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(23)00082-6",
"article-title": "Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: a phases 3, multicentre, open-label, randomised controlled trial",
"author": "Annane",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(23)00376-4_bib24",
"year": "2023"
},
{
"DOI": "10.1186/s12931-022-02126-2",
"article-title": "Efficacy and safety of the investigational complement C5 inhibitor zilucoplan in patients hospitalized with COVID-19: an open-label randomized controlled trial",
"author": "De Leeuw",
"doi-asserted-by": "crossref",
"first-page": "202",
"journal-title": "Respir Res",
"key": "10.1016/S2213-2600(23)00376-4_bib25",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1097/CCM.0000000000005683",
"article-title": "Avdoralimab (anti-C5aR1 mAb) versus placebo in patients with severe COVID-19: results from a randomized controlled trial (for COVID elimination [FORCE])",
"author": "Carvelli",
"doi-asserted-by": "crossref",
"first-page": "1788",
"journal-title": "Crit Care Med",
"key": "10.1016/S2213-2600(23)00376-4_bib26",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00297-1",
"article-title": "Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial",
"author": "Vlaar",
"doi-asserted-by": "crossref",
"first-page": "1137",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(23)00376-4_bib27",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.immuni.2021.05.010",
"article-title": "Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease",
"author": "Bergamaschi",
"doi-asserted-by": "crossref",
"first-page": "1257",
"journal-title": "Immunity",
"key": "10.1016/S2213-2600(23)00376-4_bib28",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1016/j.mce.2010.04.005",
"article-title": "The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights",
"author": "Coutinho",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Mol Cell Endocrinol",
"key": "10.1016/S2213-2600(23)00376-4_bib30",
"volume": "335",
"year": "2011"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260023003764"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of baricitinib or ravulizumab in adult patients with severe COVID-19 (TACTIC-R): a randomised, parallel-arm, open-label, phase 4 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"updated-by": [
{
"DOI": "10.1016/s2213-2600(24)00106-1",
"label": "Erratum",
"type": "erratum",
"updated": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:00:00Z",
"timestamp": 1714521600000
}
}
],
"volume": "11"
}