The time to return-to-work in healthcare workers with COVID-19 treated with ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2: An observational study utilizing pre-existing data from a single hospital
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2025.102669, Apr 2025
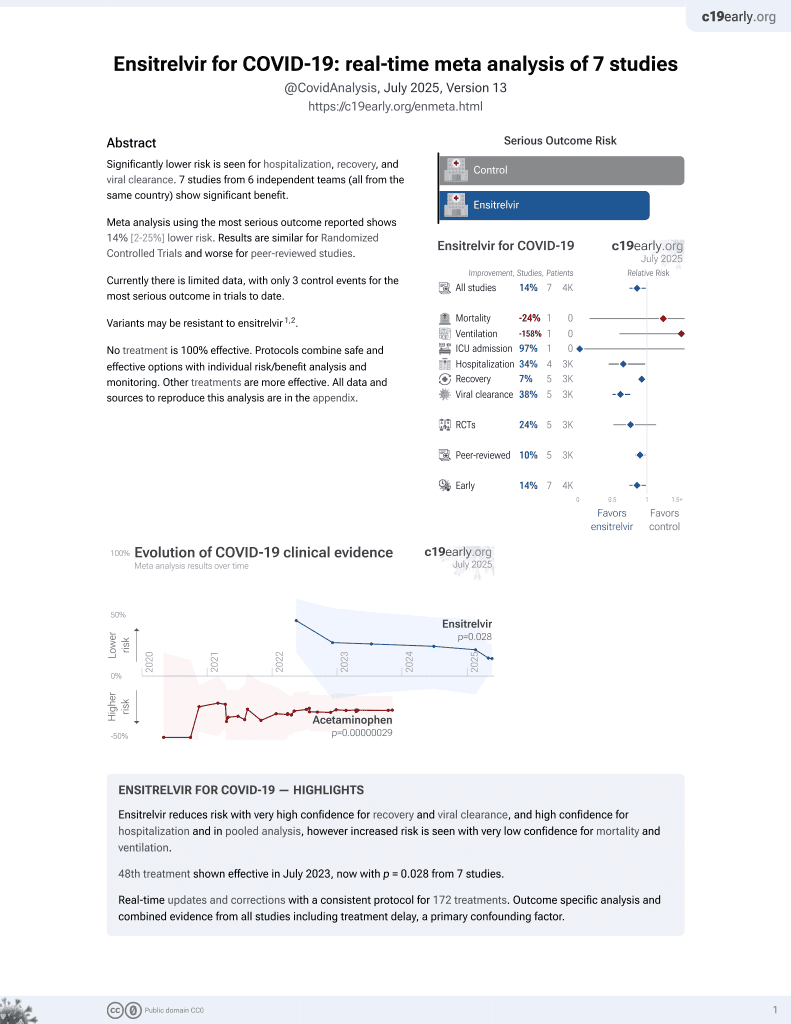
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 102 healthcare workers in Japan showing shorter time to return-to-work with ensitrelvir treatment for COVID-19.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
recovery time, 10.4% lower, relative time 0.90, p = 0.02, treatment mean 6.9 (±1.6) n=60, control mean 7.7 (±1.9) n=42.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Katsuta et al., 30 Apr 2025, retrospective, Japan, peer-reviewed, 8 authors, study period 1 June, 2023 - 30 September, 2023.
Contact: makkokatta0707@gmail.com, masatoshi.kitazono@gmail.com, naohiton918@gmail.com, yusaku.takahashi.wh@shionogi.co.jp, yasuko.ariwa@shionogi.co.jp, takuhiro.sonoyama@shionogi.co.jp, micheal@nms.ac.jp.
The time to return-to-work in healthcare workers with COVID-19 treated with ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2: An observational study utilizing pre-existing data from a single hospital
Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2025.102669
While treatment with anti-SARS-CoV-2 agents holds promise for managing healthcare workers with COVID-19, studies on this topic are limited. This study evaluated the time to return-to-work and remaining symptoms among healthcare workers with COVID-19 who received ensitrelvir and those who did not receive anti-SARS-CoV-2 drugs. This observational cohort study included healthcare workers diagnosed with COVID-19 between June and September 2023 at a single facility in Japan. Participants returned to work if they met all the following criteria: ≥5 days post-COVID-19 onset, fever resolution, and negative antigen test. The primary endpoint was the days from disease onset to return-to-work. We also evaluated the persistence of each symptom on the date of return-to-work, and the clinical and virological outcomes on the first scheduled date of return-to-work (Trial registration: UMIN000054128). The study enrolled 60 participants in the ensitrelvir group and 42 in the nonantiviral group. The mean number of days (SD) to return-to-work was 6.9 days (±1.6) in the ensitrelvir group and 7.7 days (±1.9) in the non-antiviral group. On the date of return-to-work, 4 participants in the non-antiviral group had taste disorders and 2 had smell disorders. On the first scheduled date of return-to-work (i.e. the date of first antigen test after onset), 56.7 % of participants in the ensitrelvir group and 33.3 % in the non-antiviral group had recovered, with the antigen test negativity in 76.7 % and 52.4 %, respectively. Ensitrelvir treatment for healthcare workers experiencing COVID-19 appeared to be associated with early symptom amelioration with viral load reduction, and shorter time to return-to-work.
Authorship statement Contributors M. Katsuta was the chief investigator and responsible for the organization and coordination of the trial. Y. Ariwa was responsible for the organization and coordination of the trial. Y. Takahashi was responsible for the data analysis. M. Katsuta, M. Kitazono, N. Nagai, H. Karibe and T. Yamaguchi contributed to the management or administration of the trial. T. Sonoyama and all authors developed the trial design and contributed to the writing of the final manuscript.
Declaration of competing interest Makoto Katsuda received a lecture fee from Shionogi & Co., Ltd., and Yusaku Takahashi, Yasuko Ariwa, and Takuhiro Sonoyama, are employees of Shionogi & Co., Ltd. All other authors report no potential conflicts of interest.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.jiac.2025.102669 .
References
Kase, Ota, Ota, Aoyama, Ouchi et al., Negative confirmation and infectivity estimation by quantitative antigen test of COVID-19, Jpn J of Med Tech, doi:10.14932/jamt.21-85
Mukae, Yotsuyanagi, Ohmagari, Doi, Sakaguchi et al., Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis, doi:10.1093/cid/ciac933
Nakagawa, Ogura, Hayashi, Tsukimura, Takashima, Safety and effectiveness of ensitrelvir for the treatment of COVID-19 in clinical practice -A post-marketing surveillance (interim analysis), Precis Med
O'keeffe, Constable, Chiang, Somerville, Yerramilli et al., Healthcare worker access to molnupiravir: a case series, PLoS One, doi:10.1371/journal.pone.0282695
Ohmagari, Yotsuyanagi, Doi, Yamato, Imamura et al., Efficacy and safety of ensitrelvir for asymptomatic or mild covid-19: an exploratory analysis of a multicenter, randomized, phase 2b/3 clinical trial, Influenza other respir viruses, doi:10.1111/irv.13338
Takazono, Fujita, Komeda, Miyazawa, Yoshida et al., Realworld effectiveness of ensitrelvir in reducing severe outcomes in outpatients at high risk for COVID-19, Infect Dis Ther, doi:10.1007/s40121-024-01010-4
Tsuge, Ariwa, Shibata, Pharmacological characteristics and clinical study results of ensitrelvir fumaric acid (XOCOVA(®) Tablets 125 mg)], Nihon Yakurigaku Zasshi
Yotsuyanagi, Ohmagari, Doi, Imamura, Sonoyama et al., A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part), Medicine (Baltim), doi:10.1097/MD.0000000000033024
Yotsuyanagi, Ohmagari, Doi, Yamato, Bac et al., Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial, JAMA Netw Open
Yotsuyanagi, Ohmagari, Doi, Yamato, Fukushi et al., Prevention of post COVID-19 condition by early treatment with ensitrelvir in the phase 3 SCORPIO-SR trial, Antivir Res, doi:10.1016/j.antiviral.2024.105958
Zee, Kwok, Kee, Fung, Luk et al., Impact of COVID-19 vaccination on healthcare worker infection rate and outcome during SARS-COV-2 omicron variant outbreak in Hong Kong, Vaccines, doi:10.3390/vaccines10081322
Zhuang, Zheng, Wei, Zhai, Song et al., Can the nucleic acid Ct value of discharged patients infected with SARS-CoV-2 Omicron variant be 35?-A retrospective study on fluctuation of nucleic acid Ct values in SNIEC mobile cabin hospital, Front Cell Infect Microbiol, doi:10.3389/fcimb.2022.1059880
DOI record:
{
"DOI": "10.1016/j.jiac.2025.102669",
"ISSN": [
"1341-321X"
],
"URL": "http://dx.doi.org/10.1016/j.jiac.2025.102669",
"alternative-id": [
"S1341321X25000662"
],
"article-number": "102669",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "The time to return-to-work in healthcare workers with COVID-19 treated with ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2: An observational study utilizing pre-existing data from a single hospital"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Chemotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiac.2025.102669"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Infection Prevention and Control. Published by Elsevier Ltd. All rights are reserved, including those for text and data mining, AI training, and similar technologies."
}
],
"author": [
{
"affiliation": [],
"family": "Katsuta",
"given": "Makoto",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kitazono",
"given": "Masatoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagai",
"given": "Naohito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karibe",
"given": "Hiroto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takahashi",
"given": "Yusaku",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ariwa",
"given": "Yasuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sonoyama",
"given": "Takuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamaguchi",
"given": "Tomoyoshi",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Chemotherapy",
"container-title-short": "Journal of Infection and Chemotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"jiac-j.com",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
27
]
],
"date-time": "2025-02-27T16:25:58Z",
"timestamp": 1740673558000
},
"deposited": {
"date-parts": [
[
2025,
4,
12
]
],
"date-time": "2025-04-12T02:16:43Z",
"timestamp": 1744424203000
},
"funder": [
{
"DOI": "10.13039/501100005612",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100005612",
"id-type": "DOI"
}
],
"name": "Shionogi and Co Ltd"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
12
]
],
"date-time": "2025-04-12T04:19:33Z",
"timestamp": 1744431573183,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2025,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2025,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
1
]
],
"date-time": "2025-04-01T00:00:00Z",
"timestamp": 1743465600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
1
]
],
"date-time": "2025-04-01T00:00:00Z",
"timestamp": 1743465600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
27
]
],
"date-time": "2025-02-27T00:00:00Z",
"timestamp": 1740614400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X25000662?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X25000662?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102669",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
4
]
]
},
"published-print": {
"date-parts": [
[
2025,
4
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1254/fpj.24017",
"article-title": "[Pharmacological characteristics and clinical study results of ensitrelvir fumaric acid (XOCOVA(®) Tablets 125 mg)]",
"author": "Tsuge",
"doi-asserted-by": "crossref",
"first-page": "264",
"journal-title": "Nihon Yakurigaku Zasshi",
"key": "10.1016/j.jiac.2025.102669_bib2",
"volume": "159",
"year": "2024"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"article-title": "Efficacy and safety of 5-day oral ensitrelvir for patients with mild to moderate COVID-19: the SCORPIO-SR randomized clinical trial",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiac.2025.102669_bib3",
"volume": "7",
"year": "2024"
},
{
"article-title": "Healthcare worker access to molnupiravir: a case series",
"author": "O'Keeffe",
"journal-title": "PLoS One",
"key": "10.1016/j.jiac.2025.102669_bib6",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.3389/fcimb.2022.1059880",
"article-title": "Can the nucleic acid Ct value of discharged patients infected with SARS-CoV-2 Omicron variant be 35?--A retrospective study on fluctuation of nucleic acid Ct values in SNIEC mobile cabin hospital",
"author": "Zhuang",
"doi-asserted-by": "crossref",
"journal-title": "Front Cell Infect Microbiol",
"key": "10.1016/j.jiac.2025.102669_bib7",
"volume": "12",
"year": "2022"
},
{
"article-title": "[Negative confirmation and infectivity estimation by quantitative antigen test of COVID-19]",
"author": "Kase",
"first-page": "250",
"journal-title": "Jpn J of Med Tech",
"key": "10.1016/j.jiac.2025.102669_bib8",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study",
"author": "Mukae",
"doi-asserted-by": "crossref",
"first-page": "1403",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiac.2025.102669_bib9",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1111/irv.13338",
"article-title": "Efficacy and safety of ensitrelvir for asymptomatic or mild covid-19: an exploratory analysis of a multicenter, randomized, phase 2b/3 clinical trial",
"author": "Ohmagari",
"doi-asserted-by": "crossref",
"journal-title": "Influenza other respir viruses",
"key": "10.1016/j.jiac.2025.102669_bib10",
"volume": "18",
"year": "2024"
},
{
"DOI": "10.1097/MD.0000000000033024",
"article-title": "A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part)",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"journal-title": "Medicine (Baltim)",
"key": "10.1016/j.jiac.2025.102669_bib11",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2024.105958",
"article-title": "Prevention of post COVID-19 condition by early treatment with ensitrelvir in the phase 3 SCORPIO-SR trial",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"journal-title": "Antivir Res",
"key": "10.1016/j.jiac.2025.102669_bib12",
"volume": "229",
"year": "2024"
},
{
"article-title": "Real-world effectiveness of ensitrelvir in reducing severe outcomes in outpatients at high risk for COVID-19",
"author": "Takazono",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.jiac.2025.102669_bib13",
"year": "2024"
},
{
"DOI": "10.3390/vaccines10081322",
"article-title": "Impact of COVID-19 vaccination on healthcare worker infection rate and outcome during SARS-COV-2 omicron variant outbreak in Hong Kong",
"author": "Zee",
"doi-asserted-by": "crossref",
"first-page": "1322",
"journal-title": "Vaccines",
"key": "10.1016/j.jiac.2025.102669_bib14",
"volume": "10",
"year": "2022"
},
{
"article-title": "[Safety and effectiveness of ensitrelvir for the treatment of COVID-19 in clinical practice ―A post-marketing surveillance (interim analysis)]",
"author": "Nakagawa",
"first-page": "45",
"journal-title": "Precis Med",
"key": "10.1016/j.jiac.2025.102669_bib15",
"volume": "6",
"year": "2023"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1341321X25000662"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The time to return-to-work in healthcare workers with COVID-19 treated with ensitrelvir, a novel oral inhibitor of 3C-like protease of SARS-CoV-2: An observational study utilizing pre-existing data from a single hospital",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "31"
}