A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-like Protease Inhibitor, in Japanese Patients With Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part
et al., medRxiv, doi:10.1101/2022.05.17.22275027, jRCT2031210350, May 2022
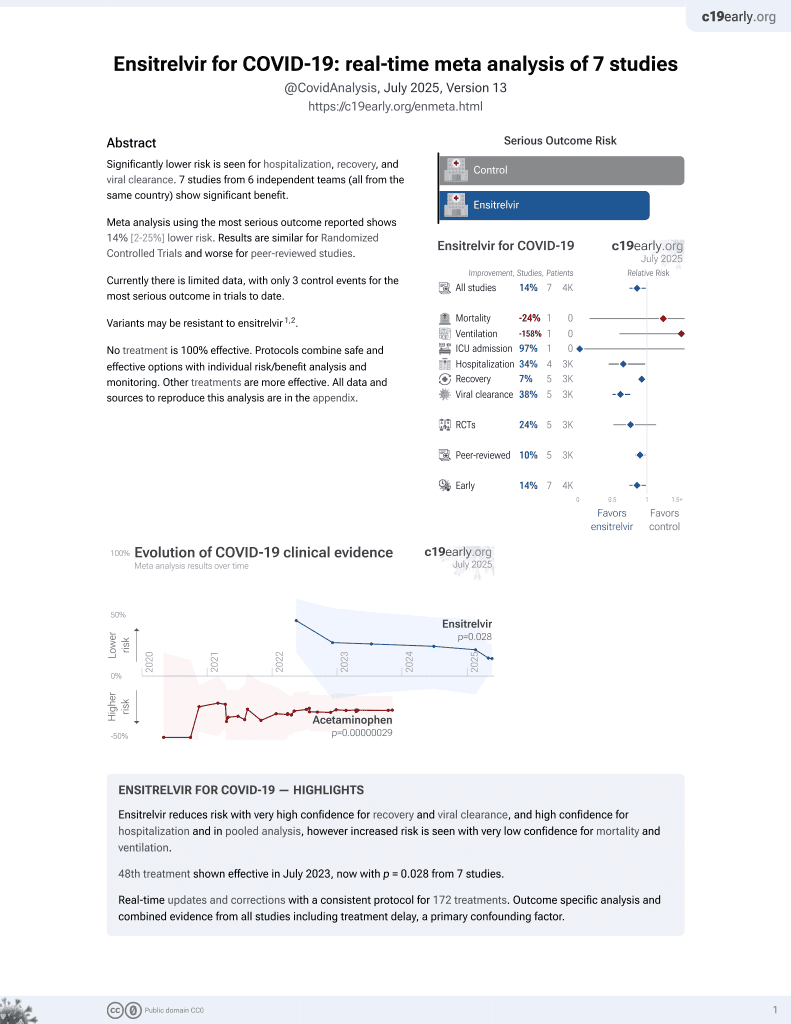
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 69 patients in in Japan, showing faster viral clearance with ensitrelvir. 5-day ensitrelvir (375mg on day 1 followed by 125 mg daily or 750mg on day 1 followed by 250mg daily).
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
relative change in viral titer, 45.2% better, RR 0.55, p = 0.002, treatment mean 2.81 (±1.21) n=14, control mean 1.54 (±0.74) n=14, day 4, 250mg, primary outcome.
|
|
relative change in viral titer, 36.4% better, RR 0.64, p = 0.048, treatment mean 2.42 (±1.42) n=15, control mean 1.54 (±0.74) n=14, day 4, 125mg, primary outcome.
|
|
time to viral-, 44.8% lower, relative time 0.55, p = 0.02, treatment 15, control 14, 125mg.
|
|
time to viral-, 43.6% lower, relative time 0.56, p = 0.02, treatment 13, control 14, 250mg.
|
|
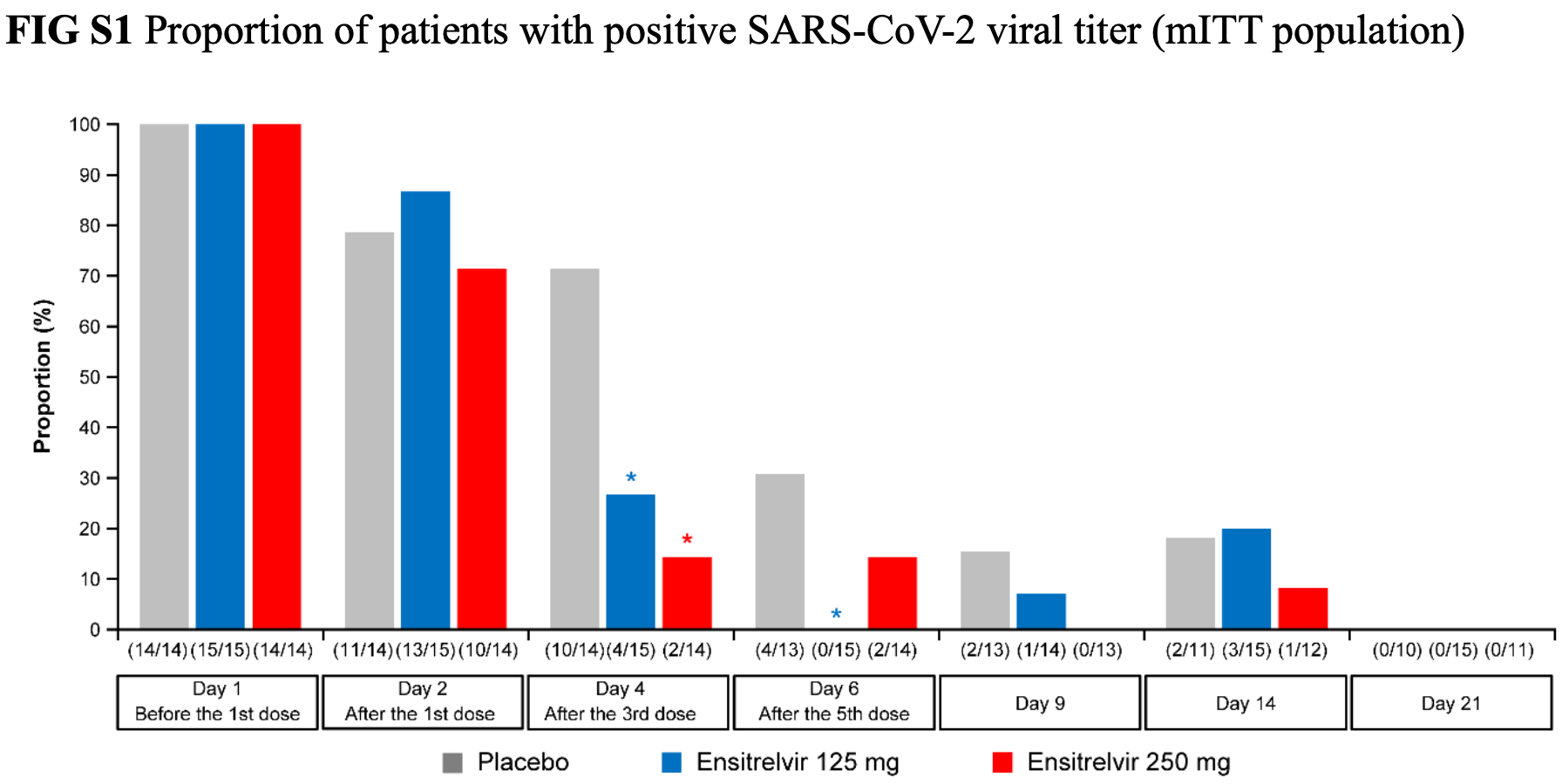
risk of no viral clearance, 54.2% lower, RR 0.46, p = 0.59, treatment 1 of 12 (8.3%), control 2 of 11 (18.2%), NNT 10, day 14, 250mg, Figure S1.
|
|
risk of no viral clearance, 10.0% higher, RR 1.10, p = 1.00, treatment 3 of 15 (20.0%), control 2 of 11 (18.2%), day 14, 125mg, Figure S1.
|
|
risk of no viral clearance, 80.0% lower, RR 0.20, p = 0.48, treatment 0 of 13 (0.0%), control 2 of 13 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 9, 250mg, Figure S1.
|
|
risk of no viral clearance, 53.6% lower, RR 0.46, p = 0.60, treatment 1 of 14 (7.1%), control 2 of 13 (15.4%), NNT 12, day 9, 125mg, Figure S1.
|
|
risk of no viral clearance, 53.6% lower, RR 0.46, p = 0.38, treatment 2 of 14 (14.3%), control 4 of 13 (30.8%), NNT 6.1, day 6, 250mg, Figure S1.
|
|
risk of no viral clearance, 89.6% lower, RR 0.10, p = 0.03, treatment 0 of 15 (0.0%), control 4 of 13 (30.8%), NNT 3.2, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 6, 125mg, Figure S1.
|
|
risk of no viral clearance, 80.0% lower, RR 0.20, p = 0.006, treatment 2 of 14 (14.3%), control 10 of 14 (71.4%), NNT 1.8, day 4, 250mg, Figure S1.
|
|
risk of no viral clearance, 62.7% lower, RR 0.37, p = 0.03, treatment 4 of 15 (26.7%), control 10 of 14 (71.4%), NNT 2.2, day 4, 125mg, Figure S1.
|
|
risk of no viral clearance, 9.1% lower, RR 0.91, p = 1.00, treatment 10 of 14 (71.4%), control 11 of 14 (78.6%), NNT 14, day 2, 250mg, Figure S1.
|
|
risk of no viral clearance, 10.3% higher, RR 1.10, p = 0.65, treatment 13 of 15 (86.7%), control 11 of 14 (78.6%), day 2, 125mg, Figure S1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mukae et al., 17 May 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, preprint, 13 authors, study period 28 September, 2021 - 1 January, 2022, trial jRCT2031210350.
Contact: takeki.uehara@shionogi.co.jp.
A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-like Protease Inhibitor, in Japanese Patients With Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part
doi:10.1101/2022.05.17.22275027
All rights reserved. No reuse allowed without permission. (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of COVID-19 -Final report, N Engl J Med
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Brown, Vostok, Johnson, Burns, Gharpure et al., Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings -Barnstable County, Massachusetts, MMWR Morb Mortal Wkly Rep
Clark, Jit, Warren-Gash, Guthrie, Wang et al., Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study, Lancet Glob Health
Fischer, Eron, Holman, Cohen, Fang et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Folgueira, Luczkowiak, Lasala, Pérez-Rivilla, Delgado, Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19, Clin Microbiol Infect
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
He, Hu, Huang, Wang, Zhang et al., Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: insights from structures of protease and inhibitors, Int J Antimicrob Agents
Mckimm-Breschkin, Hay, Cao, Cox, Dunning et al., COVID-19, influenza and RSV: surveillance-informed prevention and treatment -meeting report from an isirv-WHO virtual conference, Antiviral Res
Oran, Topol, The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review, Ann Intern Med
Singanayagam, Patel, Charlett, Bernal, Saliba et al., Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to, Euro Surveill
Unoh, Uehara, Nakahara, Nobori, Yamatsu et al., Discovery of S-217622, a non-covalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J Med Chem, doi:10.1021/acs.jmedchem.2c00117
Van Kampen, Van De Vijver, Fraaij, Haagmans, Lamers et al., Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19), Nat Commun
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with COVID-19, N Engl J Med
DOI record:
{
"DOI": "10.1101/2022.05.17.22275027",
"URL": "http://dx.doi.org/10.1101/2022.05.17.22275027",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:p>For the treatment of coronavirus disease 2019 (COVID-19), antiviral agents that can achieve rapid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reduction are warranted. This double-blind, phase 2a part of a phase 2/3 study assessed the efficacy and safety of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection. Sixty-nine patients enrolled from 56 sites were randomized (1:1:1) to orally receive 5-day ensitrelvir fumaric acid (375 mg on day 1 followed by 125 mg daily or 750 mg on day 1 followed by 250 mg daily) or placebo and followed up until day 28. The primary outcome was change from baseline in SARS-CoV-2 viral titer. A total of 16, 14, and 17 patients in the ensitrelvir 125 mg, ensitrelvir 250 mg, and placebo groups, respectively, were included in the intention-to-treat population (mean age: 38.8, 40.4, and 38.0 years, respectively). On day 4, the change from baseline in SARS-CoV-2 viral titer (log<jats:sub>10</jats:sub> 50% tissue culture infectious dose/mL) in patients with positive viral titer and viral RNA at baseline was greater with ensitrelvir 125 mg (mean [standard deviation], −2.42 [1.42]; <jats:italic>P</jats:italic> = 0.0712) and 250 mg (−2.81 [1.21]; <jats:italic>P</jats:italic> = 0.0083) versus placebo (−1.54 [0.74]), and ensitrelvir treatment reduced SARS-CoV-2 RNA by −1.4 to −1.5 log<jats:sub>10</jats:sub> copies/mL versus placebo. All adverse events were mild to moderate. Ensitrelvir treatment demonstrated rapid SARS-CoV-2 clearance and was well tolerated in patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection (Japan Registry of Clinical Trials identifier: jRCT2031210350).</jats:p>",
"accepted": {
"date-parts": [
[
2022,
5,
17
]
]
},
"author": [
{
"affiliation": [],
"family": "Mukae",
"given": "Hiroshi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yotsuyanagi",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imamura",
"given": "Takumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sonoyama",
"given": "Takuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukuhara",
"given": "Takahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ichihashi",
"given": "Genki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanaki",
"given": "Takao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baba",
"given": "Keiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takeda",
"given": "Yosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsuge",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uehara",
"given": "Takeki",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
23
]
],
"date-time": "2022-05-23T18:33:08Z",
"timestamp": 1653330788000
},
"deposited": {
"date-parts": [
[
2022,
5,
23
]
],
"date-time": "2022-05-23T18:44:59Z",
"timestamp": 1653331499000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
1,
14
]
],
"date-time": "2023-01-14T11:14:42Z",
"timestamp": 1673694882703
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2022,
5,
17
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.05.17.22275027",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
5,
17
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
5,
17
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.7326/M20-6976",
"article-title": "The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review",
"doi-asserted-by": "crossref",
"first-page": "655",
"journal-title": "Ann Intern Med",
"key": "2022052309232260000_2022.05.17.22275027v1.1",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(20)30264-3",
"article-title": "Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study",
"doi-asserted-by": "crossref",
"first-page": "e1003",
"journal-title": "Lancet Glob Health",
"key": "2022052309232260000_2022.05.17.22275027v1.2",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.32.2001483",
"doi-asserted-by": "publisher",
"key": "2022052309232260000_2022.05.17.22275027v1.3"
},
{
"DOI": "10.1016/j.cmi.2021.02.014",
"article-title": "Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19",
"doi-asserted-by": "crossref",
"first-page": "886",
"journal-title": "Clin Microbiol Infect",
"key": "2022052309232260000_2022.05.17.22275027v1.4",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-20568-4",
"doi-asserted-by": "publisher",
"key": "2022052309232260000_2022.05.17.22275027v1.5"
},
{
"DOI": "10.15585/mmwr.mm7031e2",
"article-title": "Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021",
"doi-asserted-by": "crossref",
"first-page": "1059",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2022052309232260000_2022.05.17.22275027v1.6",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2022052309232260000_2022.05.17.22275027v1.7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "2022052309232260000_2022.05.17.22275027v1.8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "2022052309232260000_2022.05.17.22275027v1.9",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/nejmoa2108163",
"doi-asserted-by": "publisher",
"key": "2022052309232260000_2022.05.17.22275027v1.10"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2022052309232260000_2022.05.17.22275027v1.11"
},
{
"DOI": "10.1101/2022.01.26.477782",
"doi-asserted-by": "crossref",
"key": "2022052309232260000_2022.05.17.22275027v1.12",
"unstructured": "Unoh Y , Uehara S , Nakahara K , Nobori H , Yamatsu Y , Yamamoto S , Maruyama Y , Taoda Y , Kasamatsu K , Suto T , Kouki K , Nakahashi A , Kawashima S , Sanaki T , Toba S , Uemura K , Mizutare T , Ando S , Sasaki M , Orba Y , Sawa H , Sato A , Sato T , Kato T , Tachibana Y. 2022. Discovery of S-217622, a non-covalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J Med Chem https://doi.org/10.1021/acs.jmedchem.2c00117."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106055",
"doi-asserted-by": "publisher",
"key": "2022052309232260000_2022.05.17.22275027v1.13"
},
{
"DOI": "10.1016/j.antiviral.2021.105227",
"article-title": "COVID-19, influenza and RSV: surveillance-informed prevention and treatment - meeting report from an isirv-WHO virtual conference",
"doi-asserted-by": "crossref",
"first-page": "105227",
"journal-title": "Antiviral Res",
"key": "2022052309232260000_2022.05.17.22275027v1.14",
"volume": "197",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"doi-asserted-by": "crossref",
"first-page": "eabl7430",
"journal-title": "Sci Transl Med",
"key": "2022052309232260000_2022.05.17.22275027v1.15",
"volume": "14",
"year": "2022"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.05.17.22275027"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-like Protease Inhibitor, in Japanese Patients With Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part",
"type": "posted-content"
}