Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2023.54991, SCORPIO-SR, jRCT2031210350, Jul 2023 (preprint)
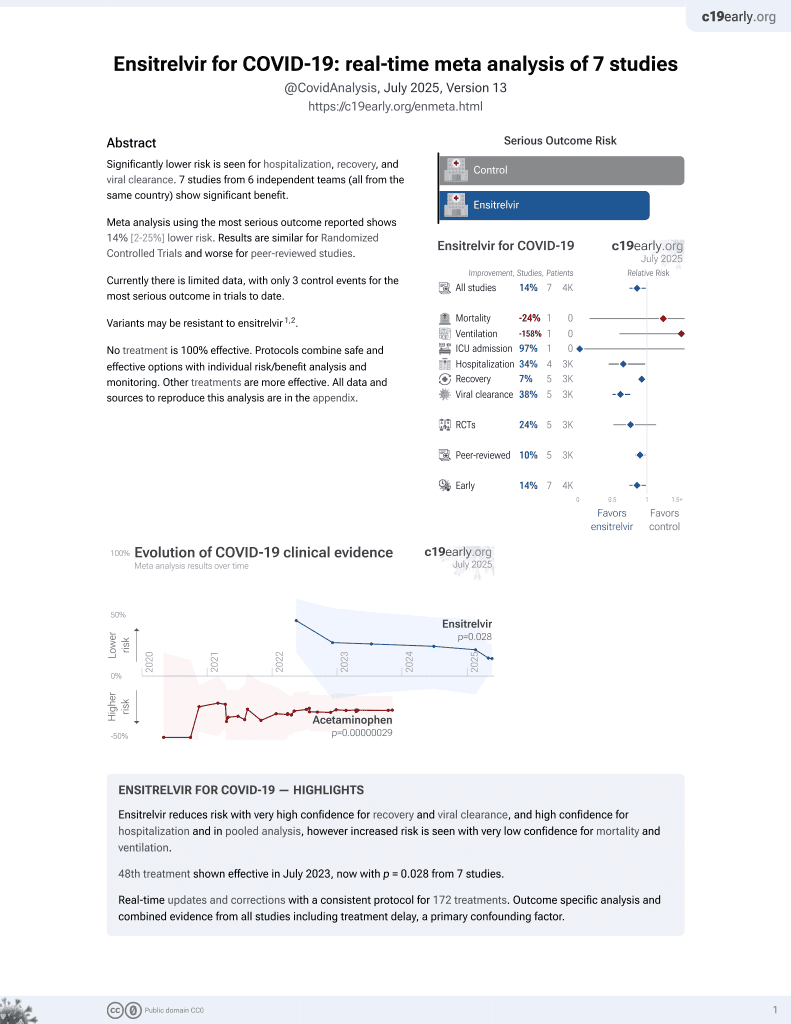
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 1,821 COVID-19 outpatients in Japan, Vietnam, and South Korea, showing improved viral clearance and improved recovery (significant for patients treated within 3 days of onset) with ensitrelvir. Only 2 hospitalizations were reported, with no deaths.
|
risk of hospitalization, 0.8% higher, RR 1.01, p = 1.00, treatment 1 of 595 (0.2%), control 1 of 600 (0.2%), 250mg.
|
|
risk of hospitalization, 66.7% lower, RR 0.33, p = 0.50, treatment 0 of 603 (0.0%), control 1 of 600 (0.2%), NNT 600, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 125mg.
|
|
recovery time, 11.4% lower, relative time 0.89, p = 0.30, treatment 577, control 572, 14 symptoms, randomized within 120 hours, 250mg.
|
|
recovery time, 10.1% lower, relative time 0.90, p = 0.46, treatment 582, control 572, 14 symptoms, randomized within 120 hours, 125mg.
|
|
recovery time, 17.9% lower, relative time 0.82, p = 0.05, treatment 330, control 321, 14 symptoms, randomized within 72 hours, 250mg.
|
|
recovery time, 19.0% lower, relative time 0.81, p = 0.03, treatment 336, control 321, 14 symptoms, randomized within 72 hours, 125mg.
|
|
recovery time, 13.3% lower, relative time 0.87, p = 0.35, treatment 577, control 572, 12 symptoms, randomized within 120 hours, 250mg.
|
|
recovery time, 9.7% lower, relative time 0.90, p = 0.76, treatment 582, control 572, 12 symptoms, randomized within 120 hours, 125mg.
|
|
recovery time, 13.3% lower, relative time 0.87, p = 0.08, treatment 330, control 321, 12 symptoms, randomized within 72 hours, 250mg.
|
|
recovery time, 15.9% lower, relative time 0.84, p = 0.07, treatment 336, control 321, 12 symptoms, randomized within 72 hours, 125mg.
|
|
relative change in viral load, 48.8% better, RR 0.51, p < 0.001, treatment 579, control 589, randomized within 120 hours, 250mg.
|
|
relative change in viral load, 48.8% better, RR 0.51, p < 0.001, treatment 592, control 589, randomized within 120 hours, 125mg.
|
|
relative change in viral load, 59.4% better, RR 0.41, p < 0.001, treatment 330, control 321, randomized within 72 hours, 250mg.
|
|
relative change in viral load, 59.3% better, RR 0.41, p < 0.001, treatment 336, control 321, randomized within 72 hours, 125mg.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yotsuyanagi et al., 13 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 35.7, 13 authors, study period 10 February, 2022 - 10 July, 2022, trial jRCT2031210350 (SCORPIO-SR).
Contact: takeki.uehara@shionogi.co.jp.
Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19
JAMA Network Open, doi:10.1001/jamanetworkopen.2023.54991
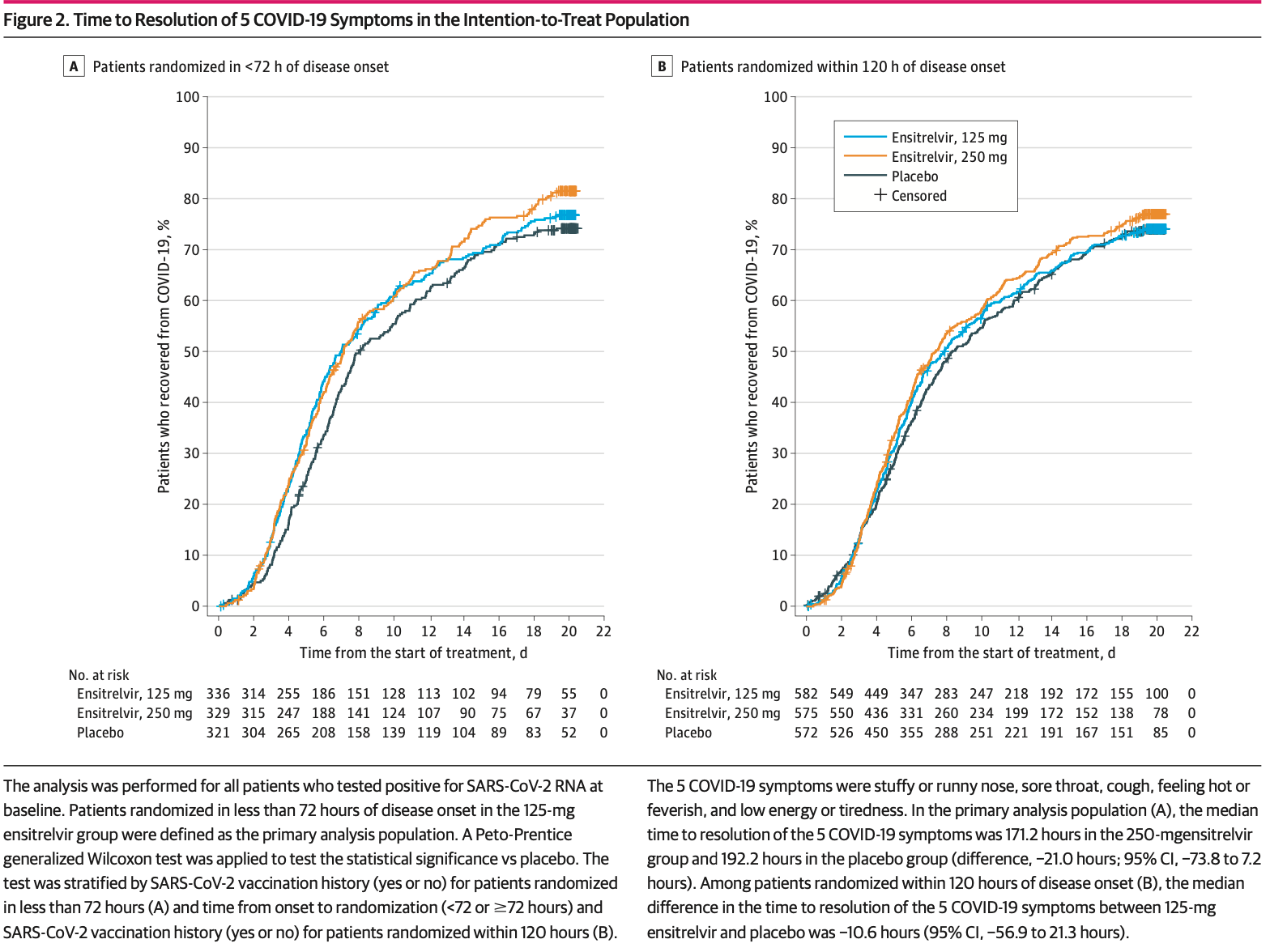
IMPORTANCE Treatment options for COVID-19 are warranted irrespective of the presence of risk factors for severe disease. OBJECTIVE To assess the efficacy and safety of ensitrelvir in patients with mild to moderate COVID-19. DESIGN, SETTING, AND PARTICIPANTS This phase 3 part of a phase 2/3, double-blind, placebocontrolled randomized clinical trial was conducted from February 10 to July 10, 2022, with a 28-day follow-up period, at 92 institutions in Japan, Vietnam, and South Korea. Patients (aged 12 to <70 years) with mild to moderate COVID-19 within 120 hours of positive viral test results were studied. INTERVENTIONS Patients were randomized (1:1:1) to receive 125 mg of once-daily ensitrelvir (375 mg on day 1), 250 mg of once-daily ensitrelvir (750 mg on day 1), or placebo for 5 days.
MAIN OUTCOMES AND MEASURES The primary end point was the time to resolution of the composite of 5 characteristic symptoms of SARS-CoV-2 Omicron infection, assessed using a Peto-Prentice generalized Wilcoxon test stratified by vaccination history. Virologic efficacy and safety were also assessed.
RESULTS A total of 1821 patients were randomized, of whom 1030 (347 in the 125-mg ensitrelvir group, 340 in the 250-mg ensitrelvir group, and 343 in the placebo group) were randomized in less than 72 hours of disease onset (primary analysis population). The mean (SD) age in this population was 35.2 (12.3) years, and 552 (53.6%) were men. A significant difference was observed between the 125-mg ensitrelvir group and the placebo group (P = .04 with a Peto-Prentice generalized Wilcoxon test). The difference in median time was approximately 1 day between the 125-mg ensitrelvir group and the placebo group (167.9 vs 192.2 hours; difference, -24.3 hours; 95% CI, -78.7 to 11.7 hours). Adverse events were observed in 267 of 604 patients (44.2%) in the 125-mg ensitrelvir group, 321 of 599 patients (53.6%) in the 250-mg ensitrelvir group, and 150 of 605 patients (24.8%) in the placebo group, which included a decrease in high-density lipoprotein level (188 [31.1%] in the 125-mg ensitrelvir group, 231 [38.6%] in the 250-mg ensitrelvir group, and 23 [3.8%] in the placebo group). No treatment-related serious adverse events were reported.
CONCLUSIONS AND RELEVANCE In this randomized clinical trial, 125-mg ensitrelvir treatment reduced the time to resolution of the 5 typical COVID-19 symptoms compared with placebo in patients treated in less than 72 hours of disease onset; the absolute difference in median time to (continued) Key Points Question Can ensitrelvir, an oral SARS-CoV-2 3C-like protease inhibitor, shorten the duration of symptoms in patients with mild to moderate COVID-19 irrespective of the presence of risk factors for severe disease? Findings In this randomized clinical trial of 1821 patients with mild to moderate COVID-19, among 1030 patients randomized in less than 72 hours of disease onset, a statistically significant difference was observed in the time to resolution..
Limitations The major limitation of this study is that it was conducted only in Asian countries, with a limited number of non-Asian patients. Also, ensitrelvir shortened the symptom duration, but the difference in median time was approximately 1 day compared with placebo. The efficacy and safety of ensitrelvir in various patient populations should be further assessed in daily clinical settings.
References
Auvigne, Vaux, Strat, Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101455
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Clinicaltrials, Gov, Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR)
Clinicaltrials, Gov, Infectious Diseases Efficacy and Safety of 5-Day Oral Ensitrelvir for Mild to Moderate COVID-19
Freidlin, Korn, Methods for accommodating nonproportional hazards in clinical trials: ready for the primary analysis?, J Clin Oncol, doi:10.1200/JCO.19.01681
Grøsland, Reme, Gjefsen, Impact of Omicron on sick leave across industries: a population-wide study, Scand J Public Health, doi:10.1177/14034948221123163
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hammond, Leister-Tebbe, Gardner, Sustained alleviation and resolution of targeted COVID-19 symptoms with nirmatrelvir/ritonavir versus placebo, Open Forum Infect Dis, doi:10.1093/ofid/ofac492.994
Hyams, Challen, Marlow, Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2022.100556
Kawashima, Matsui, Adachi, Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2023.01.040
Kopsidas, Karagiannidou, Kostaki, Global distribution, dispersal patterns, and trend of several Omicron subvariants of SARS-CoV-2 across the globe, Trop Med Infect Dis, doi:10.3390/tropicalmed7110373
Meng, Abdullahi, Ferreira, Genotype to Phenotype Japan (G2P-Japan) Consortium; Ecuador-COVID19 Consortium. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature, doi:10.1038/s41586-022-04474-x
Mukae, Yotsuyanagi, Ohmagari, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob Agents Chemother, doi:10.1128/aac.00697-22
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis, doi:10.1093/cid/ciac933
Najjar-Debbiny, Gronich, Weber, Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis, Clin Infect Dis, doi:10.1093/cid/ciac781
Nyberg, Ferguson, Nash, COVID-19 Genomics UK (COG-UK) consortium. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet, doi:10.1016/S0140-6736(22)00462-7
Sasaki, Tabata, Kishimoto, S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters, Sci Transl Med, doi:10.1126/scitranslmed.abq4064
Shimizu, Sonoyama, Fukuhara, Kuwata, Matsuo et al., Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults, Antimicrob Agents Chemother, doi:10.1128/aac.00632-22
Takashita, Yamayoshi, Simon, Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med, doi:10.1056/NEJMc2207519
Unoh, Uehara, Nakahara, Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J Med Chem, doi:10.1021/acs.jmedchem.2c00117
Uraki, Ito, Kiso, Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00070-1
Uraki, Kiso, Iida, IASO study team. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2, Nature, doi:10.1038/s41586-022-04856-1
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study, Lancet, doi:10.1016/S0140-6736(22)00017-4
Yamasoba, Kosugi, Kimura, Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00365-6
Yotsuyanagi, Ohmagari, Doi, A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part), Medicine, doi:10.1097/MD.0000000000033024
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2023.54991",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>Treatment options for COVID-19 are warranted irrespective of the presence of risk factors for severe disease.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess the efficacy and safety of ensitrelvir in patients with mild to moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>This phase 3 part of a phase 2/3, double-blind, placebo-controlled randomized clinical trial was conducted from February 10 to July 10, 2022, with a 28-day follow-up period, at 92 institutions in Japan, Vietnam, and South Korea. Patients (aged 12 to &amp;lt;70 years) with mild to moderate COVID-19 within 120 hours of positive viral test results were studied.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Patients were randomized (1:1:1) to receive 125 mg of once-daily ensitrelvir (375 mg on day 1), 250 mg of once-daily ensitrelvir (750 mg on day 1), or placebo for 5 days.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary end point was the time to resolution of the composite of 5 characteristic symptoms of SARS-CoV-2 Omicron infection, assessed using a Peto-Prentice generalized Wilcoxon test stratified by vaccination history. Virologic efficacy and safety were also assessed.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 1821 patients were randomized, of whom 1030 (347 in the 125-mg ensitrelvir group, 340 in the 250-mg ensitrelvir group, and 343 in the placebo group) were randomized in less than 72 hours of disease onset (primary analysis population). The mean (SD) age in this population was 35.2 (12.3) years, and 552 (53.6%) were men. A significant difference was observed between the 125-mg ensitrelvir group and the placebo group (<jats:italic>P</jats:italic> = .04 with a Peto-Prentice generalized Wilcoxon test). The difference in median time was approximately 1 day between the 125-mg ensitrelvir group and the placebo group (167.9 vs 192.2 hours; difference, −24.3 hours; 95% CI, −78.7 to 11.7 hours). Adverse events were observed in 267 of 604 patients (44.2%) in the 125-mg ensitrelvir group, 321 of 599 patients (53.6%) in the 250-mg ensitrelvir group, and 150 of 605 patients (24.8%) in the placebo group, which included a decrease in high-density lipoprotein level (188 [31.1%] in the 125-mg ensitrelvir group, 231 [38.6%] in the 250-mg ensitrelvir group, and 23 [3.8%] in the placebo group). No treatment-related serious adverse events were reported.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In this randomized clinical trial, 125-mg ensitrelvir treatment reduced the time to resolution of the 5 typical COVID-19 symptoms compared with placebo in patients treated in less than 72 hours of disease onset; the absolute difference in median time to resolution was approximately 1 day. Ensitrelvir demonstrated clinical and antiviral efficacy without new safety concerns. Generalizability to populations outside Asia should be confirmed.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>Japan Registry of Clinical Trials Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://jrct.niph.go.jp/en-latest-detail/jRCT2031210350\">jRCT2031210350</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "The Institute of Medical Science, The University of Tokyo, Tokyo, Japan"
}
],
"family": "Yotsuyanagi",
"given": "Hiroshi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Disease Control and Prevention Center, National Center for Global Health and Medicine, Tokyo, Japan"
}
],
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania"
},
{
"name": "Departments of Microbiology and Infectious Diseases, Fujita Health University School of Medicine, Toyoake, Japan"
}
],
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Center, Rinku General Medical Center, Izumisano, Japan"
}
],
"family": "Yamato",
"given": "Masaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Endoscopic Surgery Training Center, University Medical Center, University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam"
}
],
"family": "Bac",
"given": "Nguyen Hoang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Chung-Ang Medical Health Care System Hyundae Hospital, Gyeonggi-do, Republic of Korea"
}
],
"family": "Cha",
"given": "Bong Ki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan"
}
],
"family": "Imamura",
"given": "Takumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan"
}
],
"family": "Sonoyama",
"given": "Takuhiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan"
}
],
"family": "Ichihashi",
"given": "Genki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Division, Shionogi & Co, Ltd, Toyonaka, Japan"
}
],
"family": "Sanaki",
"given": "Takao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan"
}
],
"family": "Tsuge",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Drug Development and Regulatory Science Division, Shionogi & Co, Ltd, Osaka, Japan"
}
],
"family": "Uehara",
"given": "Takeki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan"
}
],
"family": "Mukae",
"given": "Hiroshi",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T16:31:58Z",
"timestamp": 1707496318000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T16:32:02Z",
"timestamp": 1707496322000
},
"indexed": {
"date-parts": [
[
2024,
2,
11
]
],
"date-time": "2024-02-11T11:31:30Z",
"timestamp": 1707651090869
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
2,
9
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2024,
2,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2814871/yotsuyanagi_2024_oi_231613_1706809715.91975.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e2354991",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
2,
9
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
9
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)00017-4",
"article-title": "Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study.",
"author": "Wolter",
"doi-asserted-by": "publisher",
"first-page": "437",
"issue": "10323",
"journal-title": "Lancet",
"key": "zoi231613r2",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2022.101455",
"article-title": "Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study.",
"author": "Auvigne",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "zoi231613r3",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"article-title": "Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study.",
"author": "Nyberg",
"doi-asserted-by": "publisher",
"first-page": "1303",
"issue": "10332",
"journal-title": "Lancet",
"key": "zoi231613r4",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1177/14034948221123163",
"article-title": "Impact of Omicron on sick leave across industries: a population-wide study.",
"author": "Grøsland",
"doi-asserted-by": "publisher",
"first-page": "759",
"issue": "5",
"journal-title": "Scand J Public Health",
"key": "zoi231613r5",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.3390/tropicalmed7110373",
"article-title": "Global distribution, dispersal patterns, and trend of several Omicron subvariants of SARS-CoV-2 across the globe.",
"author": "Kopsidas",
"doi-asserted-by": "publisher",
"first-page": "373",
"issue": "11",
"journal-title": "Trop Med Infect Dis",
"key": "zoi231613r6",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "zoi231613r8",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients.",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "zoi231613r9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "zoi231613r10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"article-title": "Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants.",
"author": "Takashita",
"doi-asserted-by": "publisher",
"first-page": "468",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "zoi231613r11",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00365-6",
"article-title": "Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies.",
"author": "Yamasoba",
"doi-asserted-by": "publisher",
"first-page": "942",
"issue": "7",
"journal-title": "Lancet Infect Dis",
"key": "zoi231613r12",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"article-title": "Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity.",
"author": "Meng",
"doi-asserted-by": "publisher",
"first-page": "706",
"issue": "7902",
"journal-title": "Nature",
"key": "zoi231613r13",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1021/acs.jmedchem.2c00117",
"article-title": "Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19.",
"author": "Unoh",
"doi-asserted-by": "publisher",
"first-page": "6499",
"issue": "9",
"journal-title": "J Med Chem",
"key": "zoi231613r14",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04856-1",
"article-title": "Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2.",
"author": "Uraki",
"doi-asserted-by": "publisher",
"first-page": "119",
"issue": "7917",
"journal-title": "Nature",
"key": "zoi231613r15",
"volume": "607",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abq4064",
"article-title": "S-217622, a SARS-CoV-2 main protease inhibitor, decreases viral load and ameliorates COVID-19 severity in hamsters.",
"author": "Sasaki",
"doi-asserted-by": "publisher",
"issue": "679",
"journal-title": "Sci Transl Med",
"key": "zoi231613r16",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.bbrc.2023.01.040",
"article-title": "Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally.",
"author": "Kawashima",
"doi-asserted-by": "publisher",
"first-page": "132",
"journal-title": "Biochem Biophys Res Commun",
"key": "zoi231613r17",
"volume": "645",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00070-1",
"article-title": "Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate.",
"author": "Uraki",
"doi-asserted-by": "publisher",
"first-page": "402",
"issue": "4",
"journal-title": "Lancet Infect Dis",
"key": "zoi231613r18",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1128/aac.00697-22",
"article-title": "A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part.",
"author": "Mukae",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "Antimicrob Agents Chemother",
"key": "zoi231613r19",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study.",
"author": "Mukae",
"doi-asserted-by": "publisher",
"first-page": "1403",
"issue": "8",
"journal-title": "Clin Infect Dis",
"key": "zoi231613r20",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1001/jama.2013.281053",
"article-title": "World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.",
"author": "World Medical Association",
"doi-asserted-by": "publisher",
"first-page": "2191",
"issue": "20",
"journal-title": "JAMA",
"key": "zoi231613r21",
"volume": "310",
"year": "2013"
},
{
"DOI": "10.1097/MD.0000000000033024",
"article-title": "A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part).",
"author": "Yotsuyanagi",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "Medicine (Baltimore)",
"key": "zoi231613r22",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1200/JCO.19.01681",
"article-title": "Methods for accommodating nonproportional hazards in clinical trials: ready for the primary analysis?",
"author": "Freidlin",
"doi-asserted-by": "publisher",
"first-page": "3455",
"issue": "35",
"journal-title": "J Clin Oncol",
"key": "zoi231613r25",
"volume": "37",
"year": "2019"
},
{
"DOI": "10.1128/aac.00632-22",
"article-title": "Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults.",
"author": "Shimizu",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "Antimicrob Agents Chemother",
"key": "zoi231613r26",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1016/j.lanepe.2022.100556",
"article-title": "Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom.",
"author": "Hyams",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Eur",
"key": "zoi231613r27",
"volume": "25",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial.",
"author": "Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "zoi231613r28",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac781",
"article-title": "Effectiveness of molnupiravir in high-risk patients: a propensity score matched analysis.",
"author": "Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "453",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "zoi231613r29",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac492.994",
"author": "Hammond",
"doi-asserted-by": "publisher",
"key": "zoi231613r31",
"year": "2022"
},
{
"key": "zoi231613r1",
"unstructured": "World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Accessed October 2, 2023. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern"
},
{
"key": "zoi231613r7",
"unstructured": "World Health Organization. TAG-VE statement on Omicron sublineages BQ.1 and XBB. Accessed October 2, 2023. https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb"
},
{
"key": "zoi231613r23",
"unstructured": "US Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Accessed October 2, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs"
},
{
"key": "zoi231613r24",
"unstructured": "Hiroshima Prefectural Health and Welfare Bureau. Findings from the analysis of J-SPEED (Surveillance in Post Extreme Emergencies and Disasters, Japan version) data: wave 7 analysis. Accessed October 2, 2023. https://www.mhlw.go.jp/content/10900000/000975398.pdf"
},
{
"key": "zoi231613r30",
"unstructured": "ClinicalTrials.gov. Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR). NCT05011513. Accessed October 2, 2023. https://clinicaltrials.gov/study/NCT05011513"
},
{
"key": "zoi231613r32",
"unstructured": "US Food and Drug Administration. Meeting of the Antimicrobial Drugs Advisory Committee March 16, 2023: FDA Briefing Document. Accessed October 2, 2023. https://www.fda.gov/media/166197/download"
},
{
"key": "zoi231613r33",
"unstructured": "ClinicalTrials.gov. A Study to Compare S-217622 With Placebo in Non-Hospitalized Participants With COVID-19 (SCORPIO-HR). NCT05305547. Accessed October 2, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05305547"
},
{
"key": "zoi231613r34",
"unstructured": "ClinicalTrials.gov. Strategies and Treatments for Respiratory Infections &; Viral Emergencies (STRIVE): Shionogi Protease Inhibitor. NCT05605093. Accessed October 2, 2023. https://clinicaltrials.gov/study/NCT05605093"
},
{
"key": "zoi231613r35",
"unstructured": "ClinicalTrials.gov. A Phase 3 Study of S-217622 in Pediatric Participants Aged 6 to <12 With SARS-CoV-2 Infection. jRCT2031230140. Accessed October 2, 2023. https://jrct.niph.go.jp/en-latest-detail/jRCT2031230140"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2814871"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"The SCORPIO-SR Randomized Clinical Trial"
],
"title": "Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19",
"type": "journal-article",
"volume": "7"
}