Effectiveness of Molnupiravir in High Risk Patients: a Propensity Score Matched Analysis
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac781, Sep 2022
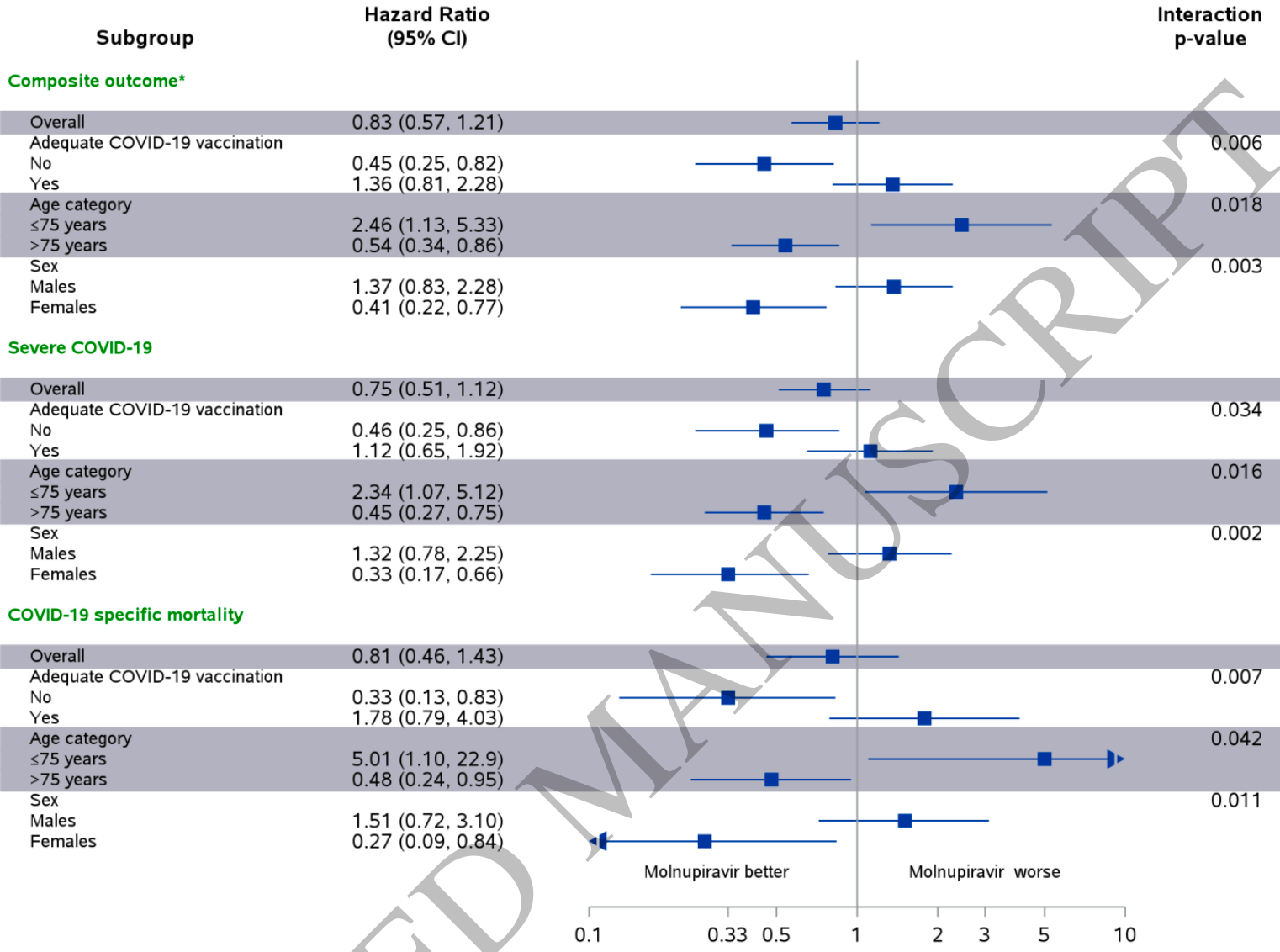
PSM retrospective 2,661 molnupiravir patients in Israel, showing lower mortality and severe COVID-19, without statistical significance. Significant benefit was seen in some subgroups, and significant harm was seen in the <75 subgroup.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
risk of death, 19.0% lower, HR 0.81, p = 0.48, treatment 22 of 2,661 (0.8%), control 27 of 2,661 (1.0%), NNT 532, propensity score matching.
|
|
risk of progression, 17.0% lower, HR 0.83, p = 0.34, treatment 50 of 2,661 (1.9%), control 60 of 2,661 (2.3%), NNT 266, severe COVID-19 or COVID-19 mortality, propensity score matching.
|
|
risk of severe case, 25.0% lower, HR 0.75, p = 0.15, treatment 43 of 2,661 (1.6%), control 57 of 2,661 (2.1%), NNT 190, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Najjar-Debbiny et al., 20 Sep 2022, retrospective, Israel, peer-reviewed, 8 authors, study period January 2022 - February 2022.
Contact: ronzana@clalit.org.il, ronza.najjar@gmail.com, saliba_wa@clalit.org.il, salibuss@technion.ac.il.
Effectiveness of Molnupiravir in High Risk Patients: a Propensity Score Matched Analysis
Background: Molnupiravir was granted emergency use authorization for the treatment of mild to moderate . In this study we used population-based real-world data to evaluate the effectiveness of Molnupiravir.
Methods: The database of the largest healthcare provider in Israel was used to identify all adults with first ever positive test for SARS-CoV-2 performed in the community during January-February 2022, who were at high risk for severe COVID-19 and had no contraindications for Molnupiravir use. Patients were included regardless of SARS-CoV-2 vaccination status. A total of 2661 patients who received Molnupiravir were propensity score-matched with 2661 patients who have not received Molnupiravir (control group). Patients were followed through 10 March 2022 for up to 28 days for the first occurrence of the composite severe COVID-19 or COVID-19 specific mortality.
Results: The composite outcome occurred in 50 patients in the Molnupiravir group and 60 patients in the control group. Molnupiravir was associated with a nonsignificant reduced risk of the composite outcome HR, 0.83 (95% CI, 0.57-1.21). However, subgroup analyses showed that Molnupiravir was associated with a significant decrease in the risk of the composite outcome in older patients 0.54 (0.34-0.86), in females 0.41 (0.22-0.77), and in patients with inadequate COVID-19 vaccination 0.45 (0.25-0.82). The results were similar when each component of the composite outcome were examined separately.
Conclusions: This study suggests that in the era of omicron and in real life setting Molnupiravir might be effective in reducing the risk of severe COVID-19 and COVID-19 related mortality, particularly in specific subgroups.
Conflict of interest The authors report no potential conflicts of interest.
A C C E P T E D
References
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
Barda, Dagan, Ben-Shlomo, Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting, N Engl J Med, doi:10.1056/NEJMoa2110475
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Doweck, Yanir, Najjar-Debbiny, Shibli, Saliba, Sudden Sensorineural Hearing Loss During the COVID-19 Pandemic, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2021.4105
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Imran, Arora, Asdaq, Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19, Molecules, doi:10.3390/molecules26195795
Menéndez-Arias, Decoding molnupiravir-induced mutagenesis in SARS-CoV-2, J Biol Chem, doi:10.1016/j.jbc.2021.100867
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients
Rosenberg, Holtgrave, Dorabawila, New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status -New York, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7034e1
Tenforde, Self, Naioti, Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults -United States, March, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7034e2
DOI record:
{
"DOI": "10.1093/cid/ciac781",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac781",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Molnupiravir was granted emergency use authorization for the treatment of mild to moderate coronavirus disease 2019 (COVID-19). In this study, we used population-based real-world data to evaluate the effectiveness of molnupiravir.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The database of the largest healthcare provider in Israel was used to identify all adults with first-ever positive test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) performed in the community during January–February 2022, who were at high risk for severe COVID-19, and had no contraindications for molnupiravir use. Patients were included regardless of SARS-CoV-2 vaccination status. A total of 2661 patients who received molnupiravir were propensity score matched with 2661 patients who have not received molnupiravir (control group). Patients were followed through 10 March 2022 for up to 28 days for the first occurrence of the composite severe COVID-19 or COVID-19-specific mortality.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The composite outcome occurred in 50 patients in the molnupiravir group and 60 patients in the control group. Molnupiravir was associated with a nonsignificant reduced risk of the composite outcome: hazard ratio, 0.83 (95% confidence interval, .57–1.21). However, subgroup analyses showed that molnupiravir was associated with a significant decrease in the risk of the composite outcome in older patients 0.54 (0.34–0.86), in females 0.41 (0.22–0.77), and in patients with inadequate COVID-19 vaccination 0.45 (0.25–0.82). The results were similar when each component of the composite outcome was examined separately.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>This study suggests that in the era of Omicron and in real-life setting, molnupiravir might be effective in reducing the risk of severe COVID-19 and COVID-19-related mortality, particularly in specific subgroups.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7586-3714",
"affiliation": [
{
"name": "Infection Control and Prevention Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
}
],
"authenticated-orcid": false,
"family": "Najjar-Debbiny",
"given": "Ronza",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Gronich",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Infectious Diseases Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Weber",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonology Division, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Pulmonology, Critical Care and Sleep Medicine, Yale School of Medicine , New Haven, Connecticut , USA"
}
],
"family": "Khoury",
"given": "Johad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Internal Medicine C, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Amar",
"given": "Maisam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Statistical Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Stein",
"given": "Nili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Internal Medicine C, Emek Medical Center , Afula , Israel"
},
{
"name": "Pharmacology Unit, Emek Medical Center , Afula , Israel"
}
],
"family": "Goldstein",
"given": "Lee Hilary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Translational Epidemiology Unit and Research Authority, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Saliba",
"given": "Walid",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
20
]
],
"date-time": "2022-09-20T15:55:29Z",
"timestamp": 1663689329000
},
"deposited": {
"date-parts": [
[
2023,
2,
8
]
],
"date-time": "2023-02-08T17:52:36Z",
"timestamp": 1675878756000
},
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T08:26:54Z",
"timestamp": 1712132814024
},
"is-referenced-by-count": 28,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
9,
20
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
9,
20
]
]
},
"published-print": {
"date-parts": [
[
2023,
2,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
20
]
],
"date-time": "2022-09-20T00:00:00Z",
"timestamp": 1663632000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac781/46564587/ciac781.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/76/3/453/49125773/ciac781.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/76/3/453/49125773/ciac781.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "453-460",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
9,
20
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
20
]
]
},
"published-other": {
"date-parts": [
[
2023,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
2,
8
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "World Health Organization (WHO)",
"key": "2023020817104413400_ciac781-B1"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "2023020817104413400_ciac781-B2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023020817104413400_ciac781-B3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/molecules26195795",
"article-title": "Discovery, development, and patent trends on molnupiravir: a prospective oral treatment for COVID-19",
"author": "Imran",
"doi-asserted-by": "crossref",
"first-page": "5795",
"journal-title": "Molecules",
"key": "2023020817104413400_ciac781-B4",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.jbc.2021.100867",
"article-title": "Decoding molnupiravir-induced mutagenesis in SARS-CoV-2",
"author": "Menéndez-Arias",
"doi-asserted-by": "crossref",
"first-page": "100867",
"journal-title": "J Biol Chem",
"key": "2023020817104413400_ciac781-B5",
"volume": "297",
"year": "2021"
},
{
"key": "2023020817104413400_ciac781-B6"
},
{
"DOI": "10.1056/NEJMoa2110475",
"article-title": "Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting",
"author": "Barda",
"doi-asserted-by": "crossref",
"first-page": "1078",
"journal-title": "N Engl J Med",
"key": "2023020817104413400_ciac781-B7",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jamaoto.2021.4105",
"article-title": "Sudden sensorineural hearing loss during the COVID-19 pandemic",
"author": "Doweck",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "JAMA Otolaryngol Head Neck Surg",
"key": "2023020817104413400_ciac781-B8",
"volume": "148",
"year": "2022"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2023020817104413400_ciac781-B9"
},
{
"key": "2023020817104413400_ciac781-B10"
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An Introduction to propensity score methods for reducing the effects of confounding in observational studies",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Multivariate Behav Res",
"key": "2023020817104413400_ciac781-B11",
"volume": "46",
"year": "2011"
},
{
"key": "2023020817104413400_ciac781-B12"
},
{
"DOI": "10.15585/mmwr.mm7034e1",
"article-title": "New COVID-19 cases and hospitalizations among adults, by vaccination Status—New York, May 3–July 25, 2021",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"first-page": "1150",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2023020817104413400_ciac781-B13",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7034e2",
"article-title": "Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March-July 2021",
"author": "Tenforde",
"doi-asserted-by": "crossref",
"first-page": "1156",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2023020817104413400_ciac781-B14",
"volume": "70",
"year": "2021"
},
{
"article-title": "Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients",
"author": "Najjar-Debbiny",
"journal-title": "Clin Infect Dis",
"key": "2023020817104413400_ciac781-B15",
"year": "2023"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/article/76/3/453/6708264"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis",
"type": "journal-article",
"volume": "76"
}