Review of the unknown long-term cancer, reproductive, and escape variant creation risks of molnupiravir. For more discussion see1-3.
4.
Shen et al., Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection, Pharmaceutics, doi:10.3390/pharmaceutics17070832.
5.
Bacigalupo et al., Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic, Frontiers in Medicine, doi:10.3389/fmed.2023.1287542.
6.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
7.
Boretti, A., On the choice of Molnupiravir and Paxlovid as the only antivirals permitted for COVID-19 infection in Australia, Clinical and Experimental Medicine, doi:10.1007/s10238-023-01010-7.
8.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
9.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
10.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
11.
Anonymous, Treating a Pandemic Respiratory Disease with a Mutagen is a Doomsday Scenario, Authorea, doi:10.22541/au.163854323.34557301/v1.
Swanstrom et al., 3 Feb 2022, peer-reviewed, 2 authors.
Abstract: VIEWPOINT: COVID-19
Lethal mutagenesis as an antiviral strategy
Lethal mutagenesis of RNA viruses is a viable antiviral strategy but has unknown risks
By Ronald Swanstrom1 and
Raymond F. Schinazi2
V
1
Department of Biochemistry and Biophysics, University
of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
2
Laboratory of Biochemical Pharmacology, Department
of Pediatrics, Emory University School of Medicine and
Children’s Healthcare of Atlanta, Atlanta, GA, USA.
Email: risunc@med.unc.edu
SCIENCE science.org
based assay, NHC was 100 times more potent
as an inhibitor of SARS-CoV-2 than ribavirin
or favipiravir (13). Molnupiravir was efficacious in mouse models of respiratory SARSCoV and Middle East respiratory syndrome
coronavirus (MERS-CoV) infection (9), consistent with NHC having broad antiviral activity (10).
A recently reported clinical trial of molnupiravir showed a 30% reduction in hospitalization when people with symptomatic
SARS-CoV-2 infection (and at risk for more
serious disease) were treated with molnupiravir within the first 5 days of symptoms
(14). Based on these results, the US Food
and Drug Administration (FDA) has approved an emergency use authorization
(EUA) for molnupiravir to treat symptomatic SARS-CoV-2 infections. Molnupiravir
has also been approved for the treatment
of COVID-19 in the United Kingdom, and
there are expectations that it will be made
widely available around the world.
However, the antiviral strategy of lethal
mutagenesis comes with a cautionary note.
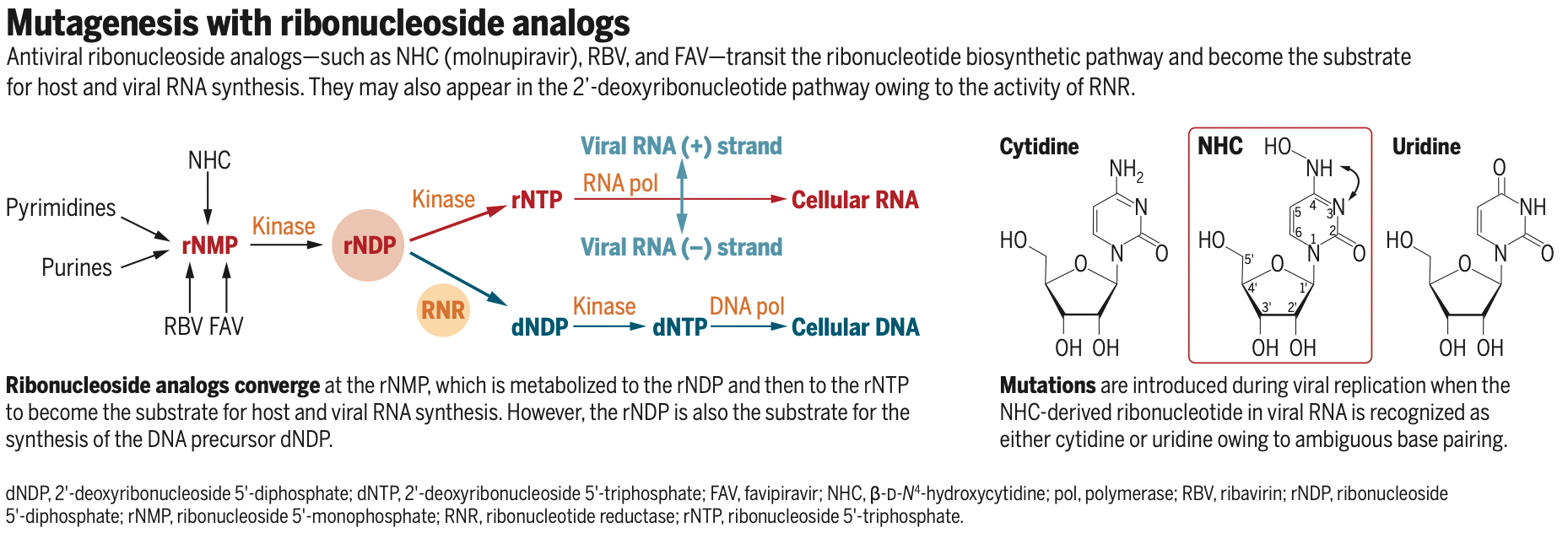
Ribonucleosides must be phosphorylated to
the 59-triphosphate form to be substrates for
RNA synthesis (host or viral). Ribonucleosides
synthesized by the host cell are formed as
the 59-monophosphate. Ribonucleoside analogs enter this biosynthetic pathway through
phosphorylation by a salvage kinase to form
the 59-monophosphate (see the figure). The
ribonucleoside 59-monophosphate is phosphorylated to the ribonucleoside 59-diphosphate and then to the 59-triphosphate (now
ready for RNA synthesis). The ribonucleoside
59-diphosphate is the obligatory intermediate in this pathway, which creates a potential
problem. Ribonucleoside 59-diphosphate is
also the obligatory intermediate in the synthesis of the 29-deoxyribonucleoside 59-diphosphate that is on the pathway to form
29-deoxyribonucleoside
59-triphosphates,
which are used in DNA synthesis. The enzyme ribonucleotide reductase (RNR) is responsible for this reaction. Thus, there is a
clear metabolic pathway for a mutagenic ribonucleoside analog to become a precursor
for host DNA synthesis.
Molnupiravir was shown to be positive in
the bacterial Ames test (an assay that measures mutagenic potential), where two animal
model assays of mutagenic potential were
largely negative, leading the FDA to state in
the EUA fact sheet that “molnupiravir is low
4 FEBRUARY 2022 • VOL 375 ISSUE 6580
497
iruses depend on the host cell to carry
out much of their replication, with
each offering only a few virus-specific
targets for the development of antiviral therapies. This makes the development of broadly active antivirals
difficult to conceptualize. Numerous RNA
viruses—including severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2), Zika
virus, and Chikungunya virus—have led to
recent epidemics, highlighting the need for
effective antiviral drugs that can be enlisted
quickly. Some years ago, a broadly applicable
antiviral strategy was proposed in..
DOI record:
{
"DOI": "10.1126/science.abn0048",
"ISSN": [
"0036-8075",
"1095-9203"
],
"URL": "http://dx.doi.org/10.1126/science.abn0048",
"abstract": "<jats:p>Lethal mutagenesis of RNA viruses is a viable a ntiviral strategy but has unknown risks</jats:p>",
"alternative-id": [
"10.1126/science.abn0048"
],
"author": [
{
"affiliation": [
{
"name": "Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA."
}
],
"family": "Swanstrom",
"given": "Ronald",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Laboratory of Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA, USA."
}
],
"family": "Schinazi",
"given": "Raymond F.",
"sequence": "additional"
}
],
"container-title": "Science",
"container-title-short": "Science",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T18:56:19Z",
"timestamp": 1643914579000
},
"deposited": {
"date-parts": [
[
2024,
1,
16
]
],
"date-time": "2024-01-16T00:03:24Z",
"timestamp": 1705363404000
},
"indexed": {
"date-parts": [
[
2024,
5,
9
]
],
"date-time": "2024-05-09T15:49:54Z",
"timestamp": 1715269794096
},
"is-referenced-by-count": 34,
"issue": "6580",
"issued": {
"date-parts": [
[
2022,
2,
4
]
]
},
"journal-issue": {
"issue": "6580",
"published-print": {
"date-parts": [
[
2022,
2,
4
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.science.org/doi/pdf/10.1126/science.abn0048",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "221",
"original-title": [],
"page": "497-498",
"prefix": "10.1126",
"published": {
"date-parts": [
[
2022,
2,
4
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
4
]
]
},
"publisher": "American Association for the Advancement of Science (AAAS)",
"reference": [
{
"author": "Morse S. S.",
"key": "e_1_3_2_2_2",
"unstructured": "S. S. Morse, E. Domingo, J. J. Holland, The Evolutionary Biology of Viruses (Raven Press, 1994).",
"volume-title": "The Evolutionary Biology of Viruses",
"year": "1994"
},
{
"DOI": "10.1128/JVI.02203-10",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_3_2"
},
{
"DOI": "10.7554/eLife.03753",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_4_2"
},
{
"DOI": "10.1073/pnas.111085598",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_5_2"
},
{
"DOI": "10.1002/rmv.483",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_6_2"
},
{
"DOI": "10.1073/pnas.2014441117",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_7_2"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_8_2"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_9_2"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_10_2"
},
{
"DOI": "10.1128/AAC.47.1.244-254.2003",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_11_2"
},
{
"DOI": "10.1039/j39680001925",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_12_2"
},
{
"DOI": "10.1016/j.jbc.2021.100770",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_13_2"
},
{
"DOI": "10.1093/infdis/jiab247",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_14_2"
},
{
"key": "e_1_3_2_15_2",
"unstructured": "A. Jayk Bernal N. Engl. J. Med. 10.1056/NEJMoa2116044 (2021)."
},
{
"key": "e_1_3_2_16_2",
"unstructured": "FDA Fact sheet for healthcare providers: Emergency use authorization for molnupiravir (2021); www.fda.gov/media/155054/download."
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.science.org/doi/10.1126/science.abn0048"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Lethal mutagenesis as an antiviral strategy",
"type": "journal-article",
"volume": "375"
}