Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection
et al., Pharmaceutics, doi:10.3390/pharmaceutics17070832, Jun 2025
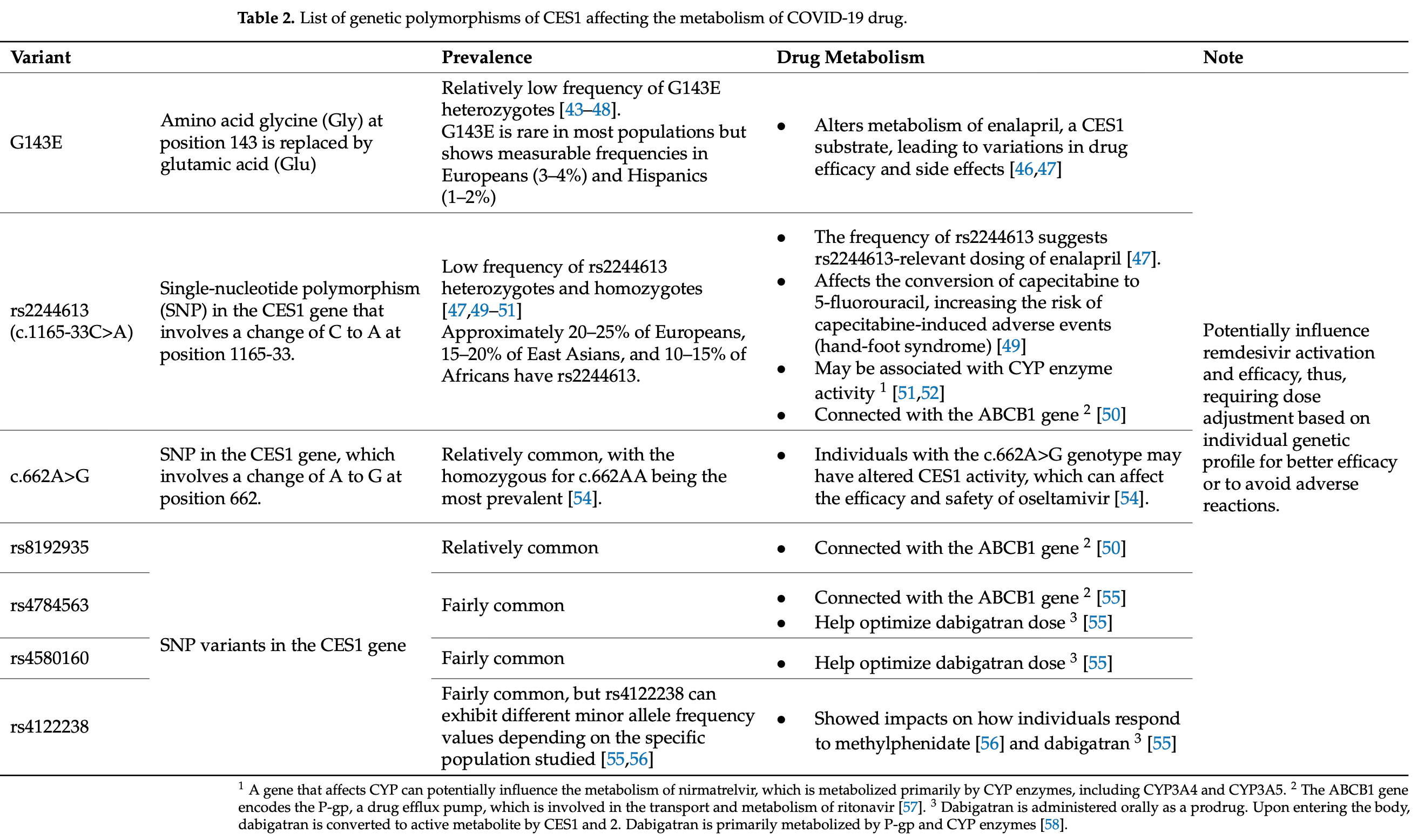
Review of how carboxylesterase (CES) enzymes influence the activity of certain COVID-19 antivirals, focusing on remdesivir, molnupiravir, nirmatrelvir, and favipiravir. Authors highlight that remdesivir requires activation by CES1, while molnupiravir is activated by CES2, and remdesivir can inhibit CES2 at nanomolar concentrations. Genetic polymorphisms in CES enzymes may significantly impact drug efficacy - for example, the G143E variant impairs CES1 function, potentially reducing remdesivir activation. Authors discusses how factors including enzyme expression patterns across tissues, drug transporters, and genetic variations can create interindividual variability in treatment response, and suggest that pharmacogenomic testing for CES variants could help optimize treatment selection.
1.
Shen et al., Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection, Pharmaceutics, doi:10.3390/pharmaceutics17070832.
2.
Bacigalupo et al., Unveiling patenting strategies of therapeutics and vaccines: evergreening in the context of COVID-19 pandemic, Frontiers in Medicine, doi:10.3389/fmed.2023.1287542.
3.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
4.
Boretti, A., On the choice of Molnupiravir and Paxlovid as the only antivirals permitted for COVID-19 infection in Australia, Clinical and Experimental Medicine, doi:10.1007/s10238-023-01010-7.
5.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
6.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
7.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
8.
Anonymous, Treating a Pandemic Respiratory Disease with a Mutagen is a Doomsday Scenario, Authorea, doi:10.22541/au.163854323.34557301/v1.
Shen et al., 26 Jun 2025, peer-reviewed, 4 authors.
Contact: dinhlk@ucmail.uc.edu (corresponding author), sheny6@mail.uc.edu, eadesws@mail.uc.edu, yanbg@uc.edu.
Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection
Pharmaceutics, doi:10.3390/pharmaceutics17070832
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, remains a major global health threat. The virus enters host cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Several small-molecule antiviral drugs, including molnupiravir, favipiravir, remdesivir, and nirmatrelvir have been shown to inhibit SARS-CoV-2 replication and are approved for treating SARS-CoV-2 infections. Nirmatrelvir inhibits the viral main protease (M pro ), a key enzyme for processing polyproteins in viral replication. In contrast, molnupiravir, favipiravir, and remdesivir are prodrugs that target RNA-dependent RNA polymerase (RdRp), which is crucial for genome replication and subgenomic RNA production. However, undergoing extensive metabolism profoundly impacts their therapeutic effects. Carboxylesterases (CES) are a family of enzymes that play an essential role in the metabolism of many drugs, especially prodrugs that require activation through hydrolysis. Molnupiravir is activated by carboxylesterase-2 (CES2), while remdesivir is hydrolytically activated by CES1 but inhibits CES2. Nirmatrelvir and remdesivir are oxidized by the same cytochrome P450 (CYP) enzyme. Additionally, various transporters are involved in the uptake or efflux of these drugs and/or their metabolites. It is well established that drug-metabolizing enzymes and transporters are differentially expressed depending on the cell type, and these genes exhibit significant polymorphisms. In this review, we examine how CES-related cellular and genetic factors influence the therapeutic activities of these widely used COVID-19 medications. This article highlights implications for improving product design, targeted inhibition, and personalized medicine by exploring genetic variations and their impact on drug metabolism and efficacy.
significant challenge related to inefficient CES activation and patient-specific CES profiles, especially under COVID-19 conditions. Targeted delivery strategies, particularly those focusing on CES2-rich intestinal tissues, offer an opportunity to maximize the bioavailability and optimize the activation and metabolism of prodrugs like molnupiravir or novel remdesivir analogs with desired dosage forms and routes of administration, less frequent dosing, and reduced toxicity. Furthermore, the development of precision antiviral therapies that correspond with patient genotypes and tissue-specific expression patterns can also be made possible by shifting the focus toward cell-type-specific drug activation by integrating local enzyme expression and regulation. This strongly supports the potential of personalized medicine in antiviral therapy.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/pharmaceutics17070832/s1 , Table S1
Conflicts of Interest: The authors declare no conflict of interest.
Abbreviations The following abbreviations are used in this manuscript:
References
Akinci, Cha, Lin, Yeo, Hamilton et al., Elucidation of remdesivir cytotoxicity pathways through genome-wide CRISPR-Cas9 screening and transcriptomics, bioRxiv, doi:10.1101/2020.08.27.270819
Alamer, Alrashed, Alfaifi, Alosaimi, Alhassar et al., Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: A retrospective study with propensity score matching sensitivity analysis, Curr. Med. Res. Opin, doi:10.1080/03007995.2021.1920900
Aloor, Aradhya, Venugopal, Gopalakrishnan Nair, Suravajhala, Glycosylation in SARS-CoV-2 variants: A path to infection and recovery, Biochem. Pharmacol, doi:10.1016/j.bcp.2022.115335
Bakos, Temesszentandrási-Ambrus, Özvegy-Laczka, Gáborik, Sarkadi et al., Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters, Int. J. Mol. Sci, doi:10.3390/ijms241411237
Beyerstedt, Casaro, Rangel, COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-020-04138-6
Birkus, Bam, Willkom, Frey, Tsai et al., Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors, Antimicrob. Agents Chemother, doi:10.1128/AAC.01834-15
Bouzidi, Driouich, Klitting, Bernadin, Piorkowski et al., Generation and evaluation of protease inhibitor-resistant SARS-CoV-2 strains, Antivir. Res, doi:10.1016/j.antiviral.2024.105814
Brown, Beery, Taran, Stevens, Henzler et al., Associations between CES1 variants and dosing and adverse effects in children taking methylphenidate, Front. Pediatr, doi:10.3389/fped.2022.958622
Choe, Jeong, Kang, Yang, Lee et al., Exploration for the effect of renal function and renal replacement therapy on pharmacokinetics of remdesivir and GS-441524 in patients with COVID-19: A limited case series, Clin. Transl. Sci, doi:10.1111/cts.13194
Cox, Wolf, Lieber, Sourimant, Lin et al., Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets, Nat. Commun, doi:10.1038/s41467-021-26760-4
Cura, Sánchez-Martín, Márquez-Pete, González-Flores, Martínez-Martínez et al., Association of Single-Nucleotide Polymorphisms in Capecitabine Bioactivation Pathway with Adjuvant Therapy Safety in Colorectal Cancer Patients, Pharmaceutics, doi:10.3390/pharmaceutics15112548
De With, Van Doorn, Maasland, Mulder, Oomen-De Hoop et al., Capecitabine-induced hand-foot syndrome: A pharmacogenetic study beyond DPYD, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.114232
Deb, Reeves, Hopefl, Bejusca, ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential, Pharmaceuticals, doi:10.3390/ph14070655
Eades, Liu, Shen, Shi, Yan, Covalent CES2 Inhibitors Protect against Reduced Formation of Intestinal Organoids by the Anticancer Drug Irinotecan, Curr. Drug Metab, doi:10.2174/1389200224666221212143904
Eastman, Roth, Brimacombe, Simeonov, Shen et al., A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19, ACS Cent. Sci, doi:10.1021/acscentsci.0c00489
Eisner, Riegler-Berket, Gamez, Sagmeister, Chalhoub et al., The Crystal Structure of Mouse Ces2c, a Potential Ortholog of Human CES2, Shows Structural Similarities in Substrate Regulation and Product Release to Human CES1, Int. J. Mol. Sci, doi:10.3390/ijms232113101
Elens, Langman, Hesselink, Van Gelder, The Impact of COVID-19 on Drug Metabolism and Pharmacokinetics, Clin. Pharmacokinet
Fujiyama, Miura, Satoh, Inoue, Kagaya et al., Influence of carboxylesterase 2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients, Xenobiotica, doi:10.1080/00498250902807338
Gandhi, Klein, Robertson, Peña-Hernández, Lin et al., De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: A case report, Nat. Commun, doi:10.1038/s41467-022-29104-y
Gandhi, Mansuri, Bansod, Potential Interactions of Remdesivir with Pulmonary Drugs: A COVID-19 Perspective, SN Compr. Clin. Med, doi:10.1007/s42399-020-00462-2
Gordon, Tchesnokov, Woolner, Perry, Feng et al., Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J. Biol. Chem, doi:10.1074/jbc.RA120.013679
Gu, Ma, Zheng, Shen, Shi et al., Left Atrial Appendage Thrombus Formation in a Patient on Dabigatran Therapy Associated With ABCB1 and CES-1 Genetic Defect, Front. Pharmacol, doi:10.3389/fphar.2018.00491
Harmer, Gilbert, Borman, Clark, Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme, FEBS Lett, doi:10.1016/S0014-5793(02)03640-2
Hashemian, Pourhanifeh, Hamblin, Shahrzad, Mirzaei, RdRp inhibitors and COVID-19: Is molnupiravir a good option?, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.112517
Her, Wang, Shi, Choi, Jung et al., Effect of CES1 genetic variation on enalapril steady-state pharmacokinetics and pharmacodynamics in healthy subjects, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14888
Hodges, Markova, Chinn, Gow, Kroetz et al., Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein), Pharmacogenet Genom, doi:10.1097/FPC.0b013e3283385a1c
Hu, Huang, Yin, The cytokine storm and COVID-19, J. Med. Virol, doi:10.1002/jmv.26232
Hu, Mady Traore, Li, Yuan, He et al., Optimization of the Prodrug Moiety of Remdesivir to Improve Lung Exposure/Selectivity and Enhance Anti-SARS-CoV-2 Activity, J. Med. Chem, doi:10.1021/acs.jmedchem.2c00758
Ikonnikova, Rodina, Dmitriev, Melnikov, Kazakov et al., The Influence of the CES1 Genotype on the Pharmacokinetics of Enalapril in Patients with Arterial Hypertension, J. Pers. Med, doi:10.3390/jpm12040580
Ji, Zhang, Xu, Wang, Li et al., The impact of ABCB1 and CES1 polymorphisms on dabigatran pharmacokinetics and pharmacodynamics in patients with atrial fibrillation, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14646
Joyce, Hu, Wang, The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): An orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations, Med. Chem. Res, doi:10.1007/s00044-022-02951-6
Kabinger, Stiller, Schmitzová, Dienemann, Kokic et al., Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat. Struct. Mol. Biol, doi:10.1038/s41594-021-00651-0
Khiali, Khani, Rouy, Entezari-Maleki, Comprehensive review on molnupiravir in COVID-19: A novel promising antiviral to combat the pandemic, Future Microbiol, doi:10.2217/fmb-2021-0252
Kim, Sai, Tanaka-Kagawa, Jinno, Ozawa et al., Haplotypes and a novel defective allele of CES2 found in a Japanese population, Drug Metab. Dispos, doi:10.1124/dmd.107.015339
Ko, Rolain, Lee, Chen, Huang et al., Arguments in favour of remdesivir for treating SARS-CoV-2 infections, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105933
Kubo, Kim, Sai, Saito, Nakajima et al., Functional characterization of three naturally occurring single nucleotide polymorphisms in the CES2 gene encoding carboxylesterase 2 (HCE-2), Drug Metab. Dispos, doi:10.1124/dmd.105.005587
Laizure, Parker, Herring, Hu, Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis, Drug Metab. Dispos, doi:10.1124/dmd.113.054353
Lavie, Dubuisson, Belouzard, SARS-CoV-2 Spike Furin Cleavage Site and S2' Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner, J. Virol, doi:10.1128/jvi.00474-22
Leegwater, Moes, Bosma, Ottens, Van Der Meer et al., Population Pharmacokinetics of Remdesivir and GS-441524 in Hospitalized COVID-19 Patients, Antimicrob. Agents Chemother, doi:10.1128/aac.00254-22
Lewis, Horenstein, Ryan, O'connell, Gibson et al., The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response, Pharmacogenet Genom, doi:10.1097/FPC.0b013e32835aa8a2
Li, Cao, Li, Cong, Li et al., Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models, J. Med. Chem, doi:10.1021/acs.jmedchem.0c01929
Li, Huang, Wang, Wang, Liang et al., COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis, J. Med. Virol, doi:10.1002/jmv.25757
Li, Liclican, Xu, Pitts, Niu et al., Key Metabolic Enzymes Involved in Remdesivir Activation in Human Lung Cells, Antimicrob. Agents Chemother, doi:10.1128/AAC.00602-21
Lin, Zeng, Mai, Gao, Fang et al., Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms, Am. J. Med. Sci, doi:10.1016/j.amjms.2023.08.014
Liu, Li, Zhu, Regulation of carboxylesterases and its impact on pharmacokinetics and pharmacodynamics: An up-to-date review, Expert Opin. Drug Metab. Toxicol, doi:10.1080/17425255.2024.2348491
Liu, Wang, Tian, Cai, Predicting the Effects of CYP2C19 and Carboxylesterases on Vicagrel, a Novel P2Y12 Antagonist, by Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling Approach, Front. Pharmacol, doi:10.3389/fphar.2020.591854
Loos, Beijnen, Schinkel, The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism?, Int. J. Mol. Sci, doi:10.3390/ijms23179866
Loos, Beijnen, Schinkel, The inhibitory and inducing effects of ritonavir on hepatic and intestinal CYP3A and other drug-handling proteins, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.114636
Lyubimov, Ed, None, doi:10.1002/9780470921920.edm014
Macip, Garcia-Segura, Mestres-Truyol, Saldivar-Espinoza, Pujadas et al., A Review of the Current Landscape of SARS-CoV-2 Main Protease Inhibitors: Have We Hit the Bullseye Yet?, Int. J. Mol. Sci, doi:10.3390/ijms23010259
Markovic, Ben-Shabat, Dahan, Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products, Pharmaceutics, doi:10.3390/pharmaceutics12111031
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications, Clin. Pharmacol. Ther, doi:10.1002/cpt.2646
Maslarinou, Manolopoulos, Ragia, Pharmacogenomic-guided dosing of fluoropyrimidines beyond DPYD: Time for a polygenic algorithm?, Front. Pharmacol, doi:10.3389/fphar.2023.1184523
Miller, Mcgrath, Zorn, Ekins, Wright et al., Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites, Mol. Pharmacol, doi:10.1124/molpharm.121.000333
Nemoda, Angyal, Tarnok, Gadoros, Sasvari-Szekely, Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD, Neuropharmacology, doi:10.1016/j.neuropharm.2009.08.014
Nie, Qian, Sun, Huang, Dong et al., Multi-Omics Analyses Reveal Systemic Insights into COVID-19 Pathophysiology, Cell
Ning, Wang, Zhan, Qi, Huang et al., Gambogic acid potentiates clopidogrelinduced apoptosis and attenuates irinotecan-induced apoptosis through down-regulating human carboxylesterase 1 and -2, Xenobiotica, doi:10.3109/00498254.2015.1125560
Oh, Lee, Lee, Cho, Yoon et al., The novel carboxylesterase 1 variant c.662A>G may decrease the bioactivation of oseltamivir in humans, PLoS ONE, doi:10.1371/journal.pone.0176320
Ohmagari, Yotsuyanagi, Doi, Yamato, Imamura et al., Efficacy and Safety of Ensitrelvir for Asymptomatic or Mild COVID-19: An Exploratory Analysis of a Multicenter, Randomized, Phase 2b/3 Clinical Trial, Influenza Other Respir. Viruses, doi:10.1111/irv.13338
Painter, Holman, Bush, Almazedi, Malik et al., Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2, Antimicrob. Agents Chemother, doi:10.1128/AAC.02428-20
Peacock, Goldhill, Zhou, Baillon, Frise et al., The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets, Nat. Microbiol, doi:10.1038/s41564-021-00908-w
Pollard, Morran, Nestor-Kalinoski, The COVID-19 pandemic: A global health crisis, Physiol. Genomics, doi:10.1152/physiolgenomics.00089.2020
Pruijssers, George, Schäfer, Leist, Gralinksi et al., Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice, Cell Rep, doi:10.1016/j.celrep.2020.107940
Ratain, Greenblatt, Drug Interactions With a Short Course of Nirmatrelvir and Ritonavir: Prescribers and Patients Beware, J. Clin. Pharmacol, doi:10.1002/jcph.2060
Ravi, Cortade, Ng, Wang, Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape, Biosens. Bioelectron, doi:10.1016/j.bios.2020.112454
Rodríguez-Lopez, Ochoa, Soria-Chacartegui, Martín-Vilchez, Navares-Gómez et al., An Investigational Study on the Role of CYP2D6, CYP3A4 and UGTs Genetic Variation on Fesoterodine Pharmacokinetics in Young Healthy Volunteers, Pharmaceuticals, doi:10.3390/ph17091236
Rossi, De Araújo, De Almeida, Ribeiro-Alves, De Almeida Velozo et al., Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress, Sci. Rep, doi:10.1038/s41598-021-88944-8
Sacco, Hu, Gongora, Meilleur, Kemp et al., The P132H mutation in the main protease of Omicron SARS-CoV-2 decreases thermal stability without compromising catalysis or small-molecule drug inhibition, Cell Res, doi:10.1038/s41422-022-00640-y
Schiel, Human Carboxylesterase 2 Splice Variants: Expression, Activity, and Role in the Metabolism of Irinotecan and Capecitabine
Shen, Eades, Liu, Yan, The COVID-19 Oral Drug Molnupiravir Is a CES2 Substrate: Potential Drug-Drug Interactions and Impact of CES2 Genetic Polymorphism In Vitro, Drug Metab. Dispos, doi:10.1124/dmd.122.000918
Shen, Eades, Yan, Remdesivir potently inhibits carboxylesterase-2 through covalent modifications: Signifying strong drug-drug interactions, Fundam. Clin. Pharmacol, doi:10.1111/fcp.12643
Shen, Eades, Yan, The COVID-19 Medicine Remdesivir Is Therapeutically Activated by Carboxylesterase-1, and Excessive Hydrolysis Increases Cytotoxicity, Hepatol. Commun, doi:10.1002/hep4.1736
Shnayder, Petrova, Shesternya, Savinova, Bochanova et al., Using Pharmacogenetics of Direct Oral Anticoagulants to Predict Changes in Their Pharmacokinetics and the Risk of Adverse Drug Reactions, Biomedicines, doi:10.3390/biomedicines9050451
Sokolova, Okhina, Shtro, Klabukov, Galochkina et al., Biostability, in vivo antiviral activity against respiratory syncytial virus, and pharmacokinetic profiles of (-)-borneol esters, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2025.177567
Strizki, Gaspar, Howe, Hutchins, Mohri et al., Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance, Antimicrob. Agents Chemother, doi:10.1128/aac.00953-23
Su, Chen, Yuan, Lausted, Choi et al., Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19, Nature, doi:10.1016/j.cell.2020.10.037
Tang, Mukundan, Yang, Charpentier, Lecluyse et al., Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol, Pharmacol. Exp. Ther, doi:10.1124/jpet.106.110577
Tian, Pang, Li, Lou, An et al., Molnupiravir and Its Antiviral Activity Against COVID-19, Front. Immunol, doi:10.3389/fimmu.2022.855496
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Hagen, Padilha, Yang, Shah et al., Preclinical Pharmacokinetics and In Vitro Properties of GS-441524, a Potential Oral Drug Candidate for COVID-19 Treatment, Front. Pharmacol, doi:10.3389/fphar.2022.918083
Wang, Her, Xiao, Shi, Wu et al., Impact of carboxylesterase 1 genetic polymorphism on trandolapril activation in human liver and the pharmacokinetics and pharmacodynamics in healthy volunteers, Clin. Transl. Sci, doi:10.1111/cts.12989
Wang, Zou, Jin, Hou, Ge et al., Human carboxylesterases: A comprehensive review, Acta Pharm. Sin. B, doi:10.1016/j.apsb.2018.05.005
Wong, Lau, Au, Lau, Hung et al., Optimal timing of nirmatrelvir/ritonavir treatment after COVID-19 symptom onset or diagnosis: Target trial emulation, Nat. Commun, doi:10.1038/s41467-023-43706-0
Xiao, Shi, Thompson, Smith, Zhang et al., Physiologically-Based Pharmacokinetic Modeling to Predict Methylphenidate Exposure Affected by Interplay Among Carboxylesterase 1 Pharmacogenetics, Drug-Drug Interactions, and Sex, J. Pharm. Sci, doi:10.1016/j.xphs.2022.04.019
Xiao, Shi, Yang, Barthel, Koch et al., Carboxylesterase-2 is a highly sensitive target of the antiobesity agent orlistat with profound implications in the activation of anticancer prodrugs, Biochem. Pharmacol, doi:10.1016/j.bcp.2012.11.026
Xu, Barauskas, Kim, Babusis, Murakami et al., Off-Target In Vitro Profiling Demonstrates that Remdesivir Is a Highly Selective Antiviral Agent, Antimicrob. Agents Chemother, doi:10.1128/AAC.02237-20
Xue, Han, Wu, Wang, Mutations in the SARS-CoV-2 spike receptor binding domain and their delicate balance between ACE2 affinity and antibody evasion, Protein Cell, doi:10.1093/procel/pwae007
Yan, Carboxylesterases, Part II: Enzyme Systems Involved in Drug Metabolism and Interactions in Animals and Humans
Yan, Yan, Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir, Fundam. Clin. Pharmacol, doi:10.1111/fcp.12889
Yang, What Do We Know About Remdesivir Drug Interactions?, Clin. Transl. Sci, doi:10.1111/cts.12815
Yasri, Wiwanitki, Molnupiravir, favipiravir and other antiviral drugs with proposed potentials for management of COVID-19: A concern on antioxidant aspect, Int. J. Biochem. Mol. Biol
Zang, Gomez Castro, Mccune, Zeng, Rothlauf et al., TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes, Sci. Immunol, doi:10.1126/sciimmunol.abc3582
Zhang, Zhang, Wu, Yu, Liu et al., A second functional furin site in the SARS-CoV-2 spike protein, Emerg. Microbes Infect, doi:10.1080/22221751.2021.2014284
Zhao, Chen, Wang, Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production, Front. Mol. Biosci, doi:10.3389/fmolb.2021.629873
Zhao, He, Huang, A novel model of molnupiravir against SARS-CoV-2 replication: Accumulated RNA mutations to induce error catastrophe, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00837-4
DOI record:
{
"DOI": "10.3390/pharmaceutics17070832",
"ISSN": [
"1999-4923"
],
"URL": "http://dx.doi.org/10.3390/pharmaceutics17070832",
"abstract": "<jats:p>Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, remains a major global health threat. The virus enters host cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Several small-molecule antiviral drugs, including molnupiravir, favipiravir, remdesivir, and nirmatrelvir have been shown to inhibit SARS-CoV-2 replication and are approved for treating SARS-CoV-2 infections. Nirmatrelvir inhibits the viral main protease (Mpro), a key enzyme for processing polyproteins in viral replication. In contrast, molnupiravir, favipiravir, and remdesivir are prodrugs that target RNA-dependent RNA polymerase (RdRp), which is crucial for genome replication and subgenomic RNA production. However, undergoing extensive metabolism profoundly impacts their therapeutic effects. Carboxylesterases (CES) are a family of enzymes that play an essential role in the metabolism of many drugs, especially prodrugs that require activation through hydrolysis. Molnupiravir is activated by carboxylesterase-2 (CES2), while remdesivir is hydrolytically activated by CES1 but inhibits CES2. Nirmatrelvir and remdesivir are oxidized by the same cytochrome P450 (CYP) enzyme. Additionally, various transporters are involved in the uptake or efflux of these drugs and/or their metabolites. It is well established that drug-metabolizing enzymes and transporters are differentially expressed depending on the cell type, and these genes exhibit significant polymorphisms. In this review, we examine how CES-related cellular and genetic factors influence the therapeutic activities of these widely used COVID-19 medications. This article highlights implications for improving product design, targeted inhibition, and personalized medicine by exploring genetic variations and their impact on drug metabolism and efficacy.</jats:p>",
"alternative-id": [
"pharmaceutics17070832"
],
"author": [
{
"affiliation": [
{
"name": "Division of Pharmaceutical Sciences, James L. Winkle College of Pharmacy, University of Cincinnati, Cincinnati, OH 45229, USA"
}
],
"family": "Shen",
"given": "Yue",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-2171-6687",
"affiliation": [
{
"name": "Division of Pharmaceutical Sciences, James L. Winkle College of Pharmacy, University of Cincinnati, Cincinnati, OH 45229, USA"
}
],
"authenticated-orcid": false,
"family": "Eades",
"given": "William",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7706-9637",
"affiliation": [
{
"name": "Division of Pharmaceutical Sciences, James L. Winkle College of Pharmacy, University of Cincinnati, Cincinnati, OH 45229, USA"
}
],
"authenticated-orcid": false,
"family": "Dinh",
"given": "Linh",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0798-5792",
"affiliation": [
{
"name": "Division of Pharmaceutical Sciences, James L. Winkle College of Pharmacy, University of Cincinnati, Cincinnati, OH 45229, USA"
}
],
"authenticated-orcid": false,
"family": "Yan",
"given": "Bingfang",
"sequence": "additional"
}
],
"container-title": "Pharmaceutics",
"container-title-short": "Pharmaceutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
7,
2
]
],
"date-time": "2025-07-02T10:46:12Z",
"timestamp": 1751453172000
},
"deposited": {
"date-parts": [
[
2025,
7,
3
]
],
"date-time": "2025-07-03T04:17:31Z",
"timestamp": 1751516251000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"R01 AI172959"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/100000002",
"id-type": "DOI"
}
],
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
3
]
],
"date-time": "2025-07-03T04:40:01Z",
"timestamp": 1751517601654,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2025,
6,
26
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2025,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
26
]
],
"date-time": "2025-06-26T00:00:00Z",
"timestamp": 1750896000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4923/17/7/832/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "832",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
6,
26
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1152/physiolgenomics.00089.2020",
"article-title": "The COVID-19 pandemic: A global health crisis",
"author": "Pollard",
"doi-asserted-by": "crossref",
"first-page": "549",
"journal-title": "Physiol. Genomics",
"key": "ref_1",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1007/s10096-020-04138-6",
"article-title": "COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection",
"author": "Beyerstedt",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "ref_2",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.abc3582",
"article-title": "TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes",
"author": "Zang",
"doi-asserted-by": "crossref",
"first-page": "eabc3582",
"journal-title": "Sci. Immunol.",
"key": "ref_3",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1093/procel/pwae007",
"article-title": "Mutations in the SARS-CoV-2 spike receptor binding domain and their delicate balance between ACE2 affinity and antibody evasion",
"author": "Xue",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "Protein Cell",
"key": "ref_4",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1128/jvi.00474-22",
"article-title": "SARS-CoV-2 Spike Furin Cleavage Site and S2’ Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner",
"author": "Lavie",
"doi-asserted-by": "crossref",
"first-page": "e0047422",
"journal-title": "J. Virol.",
"key": "ref_5",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.1038/s41564-021-00908-w",
"article-title": "The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets",
"author": "Peacock",
"doi-asserted-by": "crossref",
"first-page": "899",
"journal-title": "Nat. Microbiol.",
"key": "ref_6",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2021.2014284",
"article-title": "A second functional furin site in the SARS-CoV-2 spike protein",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "182",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_7",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3389/fmolb.2021.629873",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Zhao, X., Chen, H., and Wang, H. (2021). Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production. Front. Mol. Biosci., 8."
},
{
"DOI": "10.1016/j.bcp.2022.115335",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Aloor, A., Aradhya, R., Venugopal, P., Gopalakrishnan Nair, B., and Suravajhala, R. (2022). Glycosylation in SARS-CoV-2 variants: A path to infection and recovery. Biochem. Pharmacol., 206."
},
{
"DOI": "10.3390/pharmaceutics12111031",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Markovic, M., Ben-Shabat, S., and Dahan, A. (2020). Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics, 12."
},
{
"DOI": "10.1074/jbc.RA120.013679",
"article-title": "Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "6785",
"journal-title": "J. Biol. Chem.",
"key": "ref_11",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1021/acscentsci.0c00489",
"article-title": "Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19",
"author": "Eastman",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "ACS Cent. Sci.",
"key": "ref_12",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.855496",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Tian, L., Pang, Z., Li, M., Lou, F., An, X., Zhu, S., Song, L., Tong, Y., Fan, H., and Fan, J. (2022). Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol., 13."
},
{
"DOI": "10.1016/j.biopha.2021.112517",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Hashemian, S.M.R., Pourhanifeh, M.H., Hamblin, M.R., Shahrzad, M.K., and Mirzaei, H. (2022). RdRp inhibitors and COVID-19: Is molnupiravir a good option?. Biomed. Pharmacother., 146."
},
{
"DOI": "10.1038/s41392-021-00837-4",
"article-title": "A novel model of molnupiravir against SARS-CoV-2 replication: Accumulated RNA mutations to induce error catastrophe",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "410",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_15",
"volume": "6",
"year": "2021"
},
{
"article-title": "Molnupiravir, favipiravir and other antiviral drugs with proposed potentials for management of COVID-19: A concern on antioxidant aspect",
"author": "Yasri",
"first-page": "1",
"journal-title": "Int. J. Biochem. Mol. Biol.",
"key": "ref_16",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1080/03007995.2021.1920900",
"article-title": "Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: A retrospective study with propensity score matching sensitivity analysis",
"author": "Alamer",
"doi-asserted-by": "crossref",
"first-page": "1085",
"journal-title": "Curr. Med. Res. Opin.",
"key": "ref_17",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-29104-y",
"article-title": "De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: A case report",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1547",
"journal-title": "Nat. Commun.",
"key": "ref_18",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1128/aac.00953-23",
"article-title": "Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance",
"author": "Strizki",
"doi-asserted-by": "crossref",
"first-page": "e0095323",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_19",
"volume": "68",
"year": "2024"
},
{
"DOI": "10.1038/s41594-021-00651-0",
"article-title": "Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis",
"author": "Kabinger",
"doi-asserted-by": "crossref",
"first-page": "740",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_20",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1007/s00044-022-02951-6",
"article-title": "The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): An orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations",
"author": "Joyce",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Med. Chem. Res.",
"key": "ref_21",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.1111/irv.13338",
"article-title": "Efficacy and Safety of Ensitrelvir for Asymptomatic or Mild COVID-19: An Exploratory Analysis of a Multicenter, Randomized, Phase 2b/3 Clinical Trial",
"author": "Ohmagari",
"doi-asserted-by": "crossref",
"first-page": "e13338",
"journal-title": "Influenza Other Respir. Viruses",
"key": "ref_22",
"volume": "18",
"year": "2024"
},
{
"DOI": "10.1038/s41422-022-00640-y",
"article-title": "The P132H mutation in the main protease of Omicron SARS-CoV-2 decreases thermal stability without compromising catalysis or small-molecule drug inhibition",
"author": "Sacco",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "Cell Res.",
"key": "ref_23",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2024.105814",
"article-title": "Generation and evaluation of protease inhibitor-resistant SARS-CoV-2 strains",
"author": "Bouzidi",
"doi-asserted-by": "crossref",
"first-page": "105814",
"journal-title": "Antivir. Res.",
"key": "ref_24",
"volume": "222",
"year": "2024"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "ref_25",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2020.107940",
"article-title": "Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice",
"author": "Pruijssers",
"doi-asserted-by": "crossref",
"first-page": "107940",
"journal-title": "Cell Rep.",
"key": "ref_26",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105933",
"article-title": "Arguments in favour of remdesivir for treating SARS-CoV-2 infections",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "105933",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_27",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.3390/ijms23010259",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Macip, G., Garcia-Segura, P., Mestres-Truyol, J., Saldivar-Espinoza, B., Pujadas, G., and Garcia-Vallvé, S. (2021). A Review of the Current Landscape of SARS-CoV-2 Main Protease Inhibitors: Have We Hit the Bullseye Yet?. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_29",
"volume": "112",
"year": "2022"
},
{
"key": "ref_30",
"unstructured": "Lyubimov, A.V. (2012). Carboxylesterases. Part II: Enzyme Systems Involved in Drug Metabolism and Interactions in Animals and Humans. Encyclopedia of Drug Metabolism and Interactions, John Wiley & Sons, Inc.. [1st ed.]."
},
{
"DOI": "10.1016/j.apsb.2018.05.005",
"article-title": "Human carboxylesterases: A comprehensive review",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "699",
"journal-title": "Acta Pharm. Sin. B",
"key": "ref_31",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1002/hep4.1736",
"article-title": "The COVID-19 Medicine Remdesivir Is Therapeutically Activated by Carboxylesterase-1, and Excessive Hydrolysis Increases Cytotoxicity",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1622",
"journal-title": "Hepatol. Commun.",
"key": "ref_32",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00602-21",
"article-title": "Key Metabolic Enzymes Involved in Remdesivir Activation in Human Lung Cells",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e0060221",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_33",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1124/dmd.122.000918",
"article-title": "The COVID-19 Oral Drug Molnupiravir Is a CES2 Substrate: Potential Drug-Drug Interactions and Impact of CES2 Genetic Polymorphism In Vitro",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1151",
"journal-title": "Drug Metab. Dispos.",
"key": "ref_34",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1111/fcp.12643",
"article-title": "Remdesivir potently inhibits carboxylesterase-2 through covalent modifications: Signifying strong drug-drug interactions",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "Fundam. Clin. Pharmacol.",
"key": "ref_35",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2025.177567",
"article-title": "Biostability, in vivo antiviral activity against respiratory syncytial virus, and pharmacokinetic profiles of (-)-borneol esters",
"author": "Sokolova",
"doi-asserted-by": "crossref",
"first-page": "177567",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_36",
"volume": "996",
"year": "2025"
},
{
"DOI": "10.3390/ijms232113101",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Eisner, H., Riegler-Berket, L., Gamez, C.F.R., Sagmeister, T., Chalhoub, G., Darnhofer, B., Jazleena, P.J., Birner-Gruenberger, R., Pavkov-Keller, T., and Haemmerle, G. (2022). The Crystal Structure of Mouse Ces2c, a Potential Ortholog of Human CES2, Shows Structural Similarities in Substrate Regulation and Product Release to Human CES1. Int. J. Mol. Sci., 23."
},
{
"article-title": "The Impact of COVID-19 on Drug Metabolism and Pharmacokinetics",
"author": "Elens",
"first-page": "1357",
"journal-title": "Clin. Pharmacokinet.",
"key": "ref_38",
"volume": "59",
"year": "2020"
},
{
"key": "ref_39",
"unstructured": "Gene Expression Omnibus (GEO) Database (2025, May 31). GSE150316, Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150316."
},
{
"article-title": "Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19",
"author": "Su",
"first-page": "147",
"journal-title": "Nature",
"key": "ref_40",
"volume": "588",
"year": "2020"
},
{
"article-title": "Multi-Omics Analyses Reveal Systemic Insights into COVID-19 Pathophysiology",
"author": "Nie",
"first-page": "3165",
"journal-title": "Cell",
"key": "ref_41",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1080/17425255.2024.2348491",
"article-title": "Regulation of carboxylesterases and its impact on pharmacokinetics and pharmacodynamics: An up-to-date review",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "Expert Opin. Drug Metab. Toxicol.",
"key": "ref_42",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.1097/FPC.0b013e32835aa8a2",
"article-title": "The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response",
"author": "Lewis",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Pharmacogenet Genom.",
"key": "ref_43",
"volume": "23",
"year": "2013"
},
{
"DOI": "10.1111/cts.12989",
"article-title": "Impact of carboxylesterase 1 genetic polymorphism on trandolapril activation in human liver and the pharmacokinetics and pharmacodynamics in healthy volunteers",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1380",
"journal-title": "Clin. Transl. Sci.",
"key": "ref_44",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.xphs.2022.04.019",
"article-title": "Physiologically-Based Pharmacokinetic Modeling to Predict Methylphenidate Exposure Affected by Interplay Among Carboxylesterase 1 Pharmacogenetics, Drug-Drug Interactions, and Sex",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "2606",
"journal-title": "J. Pharm. Sci.",
"key": "ref_45",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1111/bcp.14888",
"article-title": "Effect of CES1 genetic variation on enalapril steady-state pharmacokinetics and pharmacodynamics in healthy subjects",
"author": "Her",
"doi-asserted-by": "crossref",
"first-page": "4691",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_46",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.3390/jpm12040580",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Ikonnikova, A., Rodina, T., Dmitriev, A., Melnikov, E., Kazakov, R., and Nasedkina, T. (2022). The Influence of the CES1 Genotype on the Pharmacokinetics of Enalapril in Patients with Arterial Hypertension. J. Pers. Med., 12."
},
{
"DOI": "10.1016/j.neuropharm.2009.08.014",
"article-title": "Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD",
"author": "Nemoda",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Neuropharmacology",
"key": "ref_48",
"volume": "57",
"year": "2009"
},
{
"DOI": "10.1016/j.biopha.2023.114232",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "de With, M., van Doorn, L., Maasland, D.C., Mulder, T.A.M., Oomen-de Hoop, E., Mostert, B., Homs, M.Y.V., El Bouazzaoui, S., Mathijssen, R.H.J., and van Schaik, R.H.N. (2023). Capecitabine-induced hand-foot syndrome: A pharmacogenetic study beyond DPYD. Biomed. Pharmacother., 159."
},
{
"DOI": "10.1111/bcp.14646",
"article-title": "The impact of ABCB1 and CES1 polymorphisms on dabigatran pharmacokinetics and pharmacodynamics in patients with atrial fibrillation",
"author": "Ji",
"doi-asserted-by": "crossref",
"first-page": "2247",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_50",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.5772/intechopen.95966",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Shnayder, N.A., Petrova, M.M., Shesternya, P.A., Savinova, A.V., Bochanova, E.N., Zimnitskaya, O.V., Pozhilenkova, E.A., and Nasyrova, R.F. (2021). Using Pharmacogenetics of Direct Oral Anticoagulants to Predict Changes in Their Pharmacokinetics and the Risk of Adverse Drug Reactions. Biomedicines, 9."
},
{
"DOI": "10.3390/ph17091236",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Rodríguez-Lopez, A., Ochoa, D., Soria-Chacartegui, P., Martín-Vilchez, S., Navares-Gómez, M., González-Iglesias, E., Luquero-Bueno, S., Román, M., Mejía-Abril, G., and Abad-Santos, F. (2024). An Investigational Study on the Role of CYP2D6, CYP3A4 and UGTs Genetic Variation on Fesoterodine Pharmacokinetics in Young Healthy Volunteers. Pharmaceuticals, 17."
},
{
"DOI": "10.1111/fcp.12889",
"article-title": "Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "726",
"journal-title": "Fundam. Clin. Pharmacol.",
"key": "ref_53",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0176320",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Oh, J., Lee, S., Lee, H., Cho, J.Y., Yoon, S.H., Jang, I.J., Yu, K.S., and Lim, K.S. (2017). The novel carboxylesterase 1 variant c.662A>G may decrease the bioactivation of oseltamivir in humans. PLoS ONE, 12."
},
{
"DOI": "10.3389/fphar.2018.00491",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Gu, Z.C., Ma, X.W., Zheng, X.Y., Shen, L., Shi, F.H., and Li, H. (2018). Left Atrial Appendage Thrombus Formation in a Patient on Dabigatran Therapy Associated With ABCB1 and CES-1 Genetic Defect. Front. Pharmacol., 9."
},
{
"DOI": "10.3389/fped.2022.958622",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Brown, J.T., Beery, N., Taran, A., Stevens, T., Henzler, C., Badalamenti, J., Regal, R., and McCarty, C.A. (2023). Associations between CES1 variants and dosing and adverse effects in children taking methylphenidate. Front. Pediatr., 10."
},
{
"DOI": "10.1097/FPC.0b013e3283385a1c",
"article-title": "Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein)",
"author": "Hodges",
"doi-asserted-by": "crossref",
"first-page": "152",
"journal-title": "Pharmacogenet Genom.",
"key": "ref_57",
"volume": "21",
"year": "2011"
},
{
"DOI": "10.1124/dmd.113.054353",
"article-title": "Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis",
"author": "Laizure",
"doi-asserted-by": "crossref",
"first-page": "201",
"journal-title": "Drug Metab. Dispos.",
"key": "ref_58",
"volume": "42",
"year": "2014"
},
{
"DOI": "10.3109/00498254.2015.1125560",
"article-title": "Gambogic acid potentiates clopidogrel-induced apoptosis and attenuates irinotecan-induced apoptosis through down-regulating human carboxylesterase 1 and -2",
"author": "Ning",
"doi-asserted-by": "crossref",
"first-page": "816",
"journal-title": "Xenobiotica",
"key": "ref_59",
"volume": "46",
"year": "2016"
},
{
"DOI": "10.2174/1389200224666221212143904",
"article-title": "Covalent CES2 Inhibitors Protect against Reduced Formation of Intestinal Organoids by the Anticancer Drug Irinotecan",
"author": "Eades",
"doi-asserted-by": "crossref",
"first-page": "1000",
"journal-title": "Curr. Drug Metab.",
"key": "ref_60",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2020.591854",
"doi-asserted-by": "crossref",
"key": "ref_61",
"unstructured": "Liu, S., Wang, Z., Tian, X., and Cai, W. (2020). Predicting the Effects of CYP2C19 and Carboxylesterases on Vicagrel, a Novel P2Y12 Antagonist, by Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling Approach. Front. Pharmacol., 11."
},
{
"DOI": "10.1016/j.bcp.2012.11.026",
"article-title": "Carboxylesterase-2 is a highly sensitive target of the antiobesity agent orlistat with profound implications in the activation of anticancer prodrugs",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "439",
"journal-title": "Biochem. Pharmacol.",
"key": "ref_62",
"volume": "85",
"year": "2013"
},
{
"DOI": "10.1080/00498250902807338",
"article-title": "Influence of carboxylesterase 2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients",
"author": "Fujiyama",
"doi-asserted-by": "crossref",
"first-page": "407",
"journal-title": "Xenobiotica",
"key": "ref_63",
"volume": "39",
"year": "2009"
},
{
"DOI": "10.1124/dmd.107.015339",
"article-title": "Haplotypes and a novel defective allele of CES2 found in a Japanese population",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1865",
"journal-title": "Drug Metab. Dispos.",
"key": "ref_64",
"volume": "35",
"year": "2007"
},
{
"DOI": "10.3390/pharmaceutics15112548",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Cura, Y., Sánchez-Martín, A., Márquez-Pete, N., González-Flores, E., Martínez-Martínez, F., Pérez-Ramírez, C., and Jiménez-Morales, A. (2023). Association of Single-Nucleotide Polymorphisms in Capecitabine Bioactivation Pathway with Adjuvant Therapy Safety in Colorectal Cancer Patients. Pharmaceutics, 15."
},
{
"DOI": "10.3389/fphar.2023.1184523",
"doi-asserted-by": "crossref",
"key": "ref_66",
"unstructured": "Maslarinou, A., Manolopoulos, V.G., and Ragia, G. (2023). Pharmacogenomic-guided dosing of fluoropyrimidines beyond DPYD: Time for a polygenic algorithm?. Front. Pharmacol., 14."
},
{
"key": "ref_67",
"unstructured": "Schiel, M.A. (2009). Human Carboxylesterase 2 Splice Variants: Expression, Activity, and Role in the Metabolism of Irinotecan and Capecitabine. [Ph.D. Thesis, Department of Biochemistry & Molecular Biology, Indiana University]."
},
{
"DOI": "10.1124/jpet.106.110577",
"article-title": "Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "1467",
"journal-title": "Pharmacol. Exp. Ther.",
"key": "ref_68",
"volume": "319",
"year": "2006"
},
{
"DOI": "10.1124/dmd.105.005587",
"article-title": "Functional characterization of three naturally occurring single nucleotide polymorphisms in the CES2 gene encoding carboxylesterase 2 (HCE-2)",
"author": "Kubo",
"doi-asserted-by": "crossref",
"first-page": "1482",
"journal-title": "Drug Metab. Dispos.",
"key": "ref_69",
"volume": "33",
"year": "2005"
},
{
"DOI": "10.1128/AAC.01834-15",
"article-title": "Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors",
"author": "Birkus",
"doi-asserted-by": "crossref",
"first-page": "316",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_70",
"volume": "60",
"year": "2015"
},
{
"DOI": "10.1021/acs.jmedchem.0c01929",
"article-title": "Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "2785",
"journal-title": "J. Med. Chem.",
"key": "ref_71",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1038/s41467-021-26760-4",
"article-title": "Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "6415",
"journal-title": "Nat. Commun.",
"key": "ref_72",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s42399-020-00462-2",
"article-title": "Potential Interactions of Remdesivir with Pulmonary Drugs: A COVID-19 Perspective",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1707",
"journal-title": "SN Compr. Clin. Med.",
"key": "ref_73",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1111/cts.12815",
"article-title": "What Do We Know About Remdesivir Drug Interactions?",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "842",
"journal-title": "Clin. Transl. Sci.",
"key": "ref_74",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.3390/ph14070655",
"doi-asserted-by": "crossref",
"key": "ref_75",
"unstructured": "Deb, S., Reeves, A.A., Hopefl, R., and Bejusca, R. (2021). ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals, 14."
},
{
"DOI": "10.1128/aac.00254-22",
"article-title": "Population Pharmacokinetics of Remdesivir and GS-441524 in Hospitalized COVID-19 Patients",
"author": "Leegwater",
"doi-asserted-by": "crossref",
"first-page": "e0025422",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_76",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1111/cts.13194",
"article-title": "Exploration for the effect of renal function and renal replacement therapy on pharmacokinetics of remdesivir and GS-441524 in patients with COVID-19: A limited case series",
"author": "Choe",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Clin. Transl. Sci.",
"key": "ref_77",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1128/AAC.02237-20",
"article-title": "Off-Target In Vitro Profiling Demonstrates that Remdesivir Is a Highly Selective Antiviral Agent",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "e02237-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_78",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26232",
"article-title": "The cytokine storm and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "250",
"journal-title": "J. Med. Virol.",
"key": "ref_79",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1124/molpharm.121.000333",
"article-title": "Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites",
"author": "Miller",
"doi-asserted-by": "crossref",
"first-page": "548",
"journal-title": "Mol. Pharmacol.",
"key": "ref_80",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2022.918083",
"doi-asserted-by": "crossref",
"key": "ref_81",
"unstructured": "Wang, A.Q., Hagen, N.R., Padilha, E.C., Yang, M., Shah, P., Chen, C.Z., Huang, W., Terse, P., Sanderson, P., and Zheng, W. (2022). Preclinical Pharmacokinetics and In Vitro Properties of GS-441524, a Potential Oral Drug Candidate for COVID-19 Treatment. Front. Pharmacol., 13."
},
{
"DOI": "10.1101/2020.08.27.270819",
"doi-asserted-by": "crossref",
"key": "ref_82",
"unstructured": "Akinci, E., Cha, M., Lin, L., Yeo, G., Hamilton, M.C., Donahue, C.J., Bermudez-Cabrera, H.C., Zanetti, L.C., Chen, M., and Barkal, S.A. (2020). Elucidation of remdesivir cytotoxicity pathways through genome-wide CRISPR-Cas9 screening and transcriptomics. bioRxiv."
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"first-page": "e02428-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_83",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.2217/fmb-2021-0252",
"article-title": "Comprehensive review on molnupiravir in COVID-19: A novel promising antiviral to combat the pandemic",
"author": "Khiali",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "Future Microbiol.",
"key": "ref_84",
"volume": "17",
"year": "2022"
},
{
"key": "ref_85",
"unstructured": "Rossi, Á.D., de Araújo, J.L.F., de Almeida, T.B., Ribeiro-Alves, M., de Almeida Velozo, C., Almeida, J.M., de Carvalho Leitão, I., Ferreira, S.N., da Silva Oliveira, J., and Alves, H.J. (2021). Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci. Rep., 11."
},
{
"DOI": "10.1002/jmv.25757",
"article-title": "COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "577",
"journal-title": "J. Med. Virol.",
"key": "ref_86",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1016/S0014-5793(02)03640-2",
"article-title": "Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme",
"author": "Harmer",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "FEBS Lett.",
"key": "ref_87",
"volume": "532",
"year": "2002"
},
{
"DOI": "10.1016/j.amjms.2023.08.014",
"article-title": "Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Am. J. Med. Sci.",
"key": "ref_88",
"volume": "366",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.2c00758",
"article-title": "Optimization of the Prodrug Moiety of Remdesivir to Improve Lung Exposure/Selectivity and Enhance Anti-SARS-CoV-2 Activity",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "12044",
"journal-title": "J. Med. Chem.",
"key": "ref_89",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.3390/ijms241411237",
"doi-asserted-by": "crossref",
"key": "ref_90",
"unstructured": "Bakos, É., Temesszentandrási-Ambrus, C., Özvegy-Laczka, C., Gáborik, Z., Sarkadi, B., and Telbisz, Á. (2023). Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1016/j.bios.2020.112454",
"doi-asserted-by": "crossref",
"key": "ref_91",
"unstructured": "Ravi, N., Cortade, D.L., Ng, E., and Wang, S.X. (2020). Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron., 165."
},
{
"DOI": "10.1016/j.biopha.2023.114636",
"doi-asserted-by": "crossref",
"key": "ref_92",
"unstructured": "Loos, N.H.C., Beijnen, J.H., and Schinkel, A.H. (2023). The inhibitory and inducing effects of ritonavir on hepatic and intestinal CYP3A and other drug-handling proteins. Biomed. Pharmacother., 162."
},
{
"DOI": "10.3390/ijms23179866",
"doi-asserted-by": "crossref",
"key": "ref_93",
"unstructured": "Loos, N.H.C., Beijnen, J.H., and Schinkel, A.H. (2022). The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism?. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1002/jcph.2060",
"article-title": "Drug Interactions With a Short Course of Nirmatrelvir and Ritonavir: Prescribers and Patients Beware",
"author": "Ratain",
"doi-asserted-by": "crossref",
"first-page": "925",
"journal-title": "J. Clin. Pharmacol.",
"key": "ref_94",
"volume": "62",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-43706-0",
"article-title": "Optimal timing of nirmatrelvir/ritonavir treatment after COVID-19 symptom onset or diagnosis: Target trial emulation",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "8377",
"journal-title": "Nat. Commun.",
"key": "ref_95",
"volume": "14",
"year": "2023"
}
],
"reference-count": 95,
"references-count": 95,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4923/17/7/832"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection",
"type": "journal-article",
"volume": "17"
}
shen6