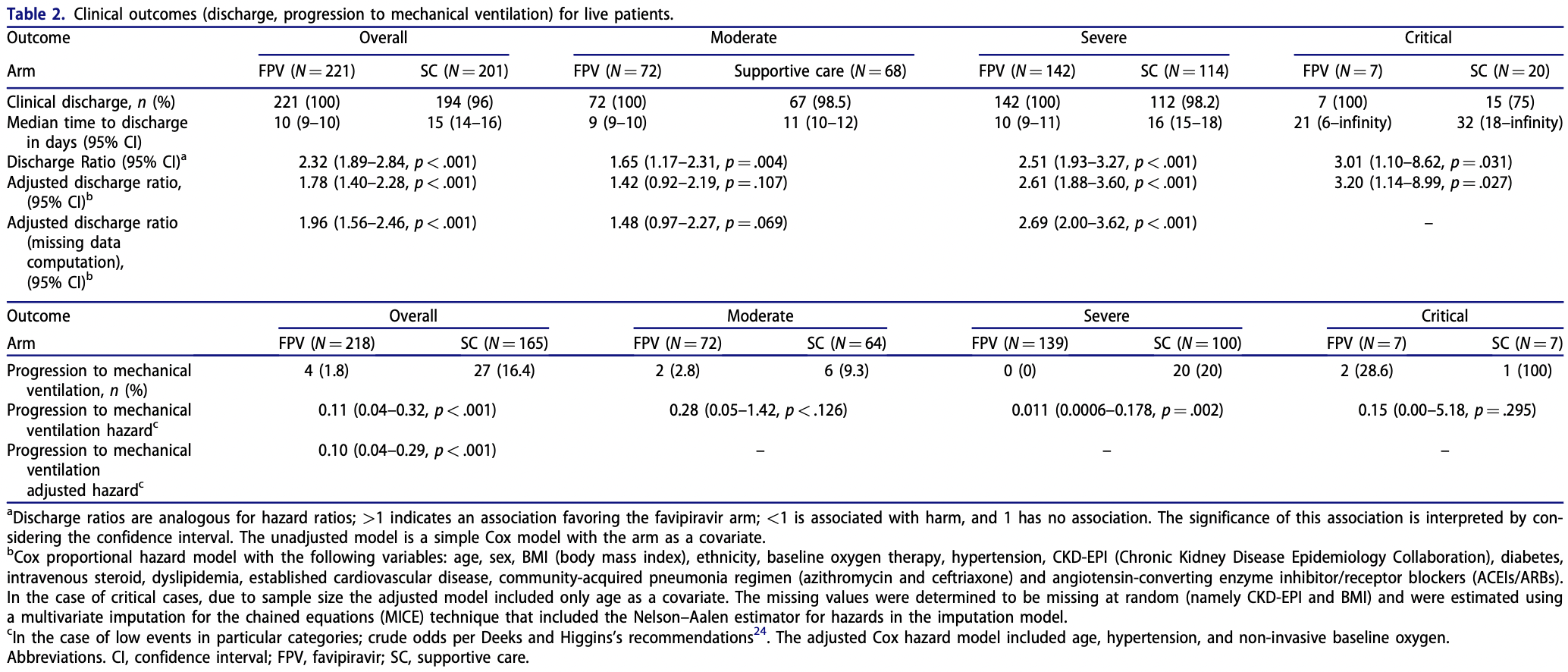
Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis
et al., Current Medical Research and Opinion, doi:10.1080/03007995.2021.1920900, May 2021
Retrospective 234 favipiravir and 223 control patients in Saudi Arabia, showing shorter time to discharge and lower progression to ventilation, but no significant difference in mortality.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 56.0% higher, HR 1.56, p = 0.26, treatment 12 of 233 (5.2%), control 21 of 223 (9.4%), adjusted per study, day 90.
|
|
risk of mechanical ventilation, 90.0% lower, HR 0.10, p < 0.001, treatment 4 of 218 (1.8%), control 27 of 165 (16.4%), NNT 6.9, adjusted per study.
|
|
adjusted discharge ratio, 49.0% lower, RR 0.51, p < 0.001, treatment 221, control 201, adjusted per study, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Alamer et al., 19 May 2021, retrospective, Saudi Arabia, peer-reviewed, 18 authors.
Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis
Current Medical Research and Opinion, doi:10.1080/03007995.2021.1920900
Introduction: Favipiravir is a repurposed drug to treat coronavirus 2019 (COVID-19). Due to a lack of available real-world data, we assessed its effectiveness and safety in moderately to critically ill COVID-19 patients. Methods: This retrospective study was conducted in two public/specialty hospitals in Saudi Arabia. We included patients !18 years) admitted April-August 2020 with confirmed SARS-CoV-2 diagnosed by real-time polymerase chain reaction (RT-PCR) from nasopharyngeal swab. Patients received either favipiravir (1800 mg or 1600 mg twice daily loading dose, followed by 800 mg or 600 mg twice daily) or supportive-care treatment. Patients were excluded if they were outside the study period, classified as having a mild form of the disease per WHO criteria, or had an incomplete patient file. Kaplan-Meier (KM) models were used to estimate median time to discharge. Discharge ratios, progression to mechanical ventilation, and mortality outcomes were estimated across the severity spectrum using Cox proportional-hazards models. As a sensitivity analysis, we performed propensity score-matching (PSM) analysis. Results: Overall, median time to discharge was 10 days (95%CI ¼ 9-10) in the favipiravir arm versus 15 days (95%CI ¼ 14-16) in the supportive-care arm. The accelerated discharge benefit was seen across the COVID-19 spectrum of severity. The adjusted discharge ratio was 1.96 (95%CI ¼ 1.56-2.46). Progression to mechanical ventilation was slower with favipiravir (HR adj ¼ 0.10, 95%CI ¼ 0.04-0.29). There was no significant effect on mortality (HR adj ¼ 1.56, 95%CI ¼ 0.73-3.36). There was a statistically non-significant trend toward worse outcomes in the critical category (HR adj ¼ 2.80, 95%CI ¼ 0.99-7.89). Age was an independent risk factor for mortality in mechanically ventilated patients. PSM analyses confirmed these findings. Conclusion: Favipiravir was associated with clinical benefits, including accelerated discharge rate and less progression to mechanical ventilation; however, no overall mortality benefits were seen across the severity spectrum.
Author contributions Conceptualization: Ahmad Alamer and Ahmad A Alrashed; methodology: Ahmad Alamer and Ivo Abraham; software: Ahmad Alamer; validation: Abdulaziz S. Alulhmim and Ivo Abraham; formal analysis: Ahmad Alamer; data curation: Fatima Alhassar, Malak M. Almutairi, Jude Howaidi, Wedad Almutairi, and Mashael Alfaifi; writing of original draft preparation: Ahmad Alamer and Abdulaziz S. Alulhmim; writing, reviewing, and editing: Ahmad Alamer, Abdulaziz S. Alulhmim, Ivo Abraham, Ahmad A Alrashed, Bandar Alosaimi, Yahya Mohzari, Tarek Sulaiman, Ahmed AlJedai, Alaa H. Alali, and Abdulla Baradwan; visualization: Ahmad Alamer; supervision: Ahmad Alamer and Ivo Abraham; project administration: Ahmad Alamer, Mashael Alfaifi, Ahmad A Alrashed, and Yahya Mohzari. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, declare their responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
References
Alamer, Al, None
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -preliminary report. Reply, N Engl J Med
Bradburn, Clark, Love, Survival analysis part II: multivariate data analysis-an introduction to concepts and methods, Br J Cancer
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Chen, Zhang, Huang, Favipiravir versus arbidol for COVID-19: a randomized clinical trial, medRxiv, doi:10.1101/2020.03.17.20037432
D'agostino, Propensity scores in cardiovascular research, Circulation
Deeks, Higgins, Altman, Analysing data and undertaking meta-analyses
Furuta, Gowen, Takahashi, Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inf
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Irie, Nakagawa, Fujita, Pharmacokinetics of favipiravir in critically ill patients with COVID-19, Clin Transl Sci
Lumley, Analysis of complex survey samples, J Stat Soft
Mitra, Reiter, A comparison of two methods of estimating propensity scores after multiple imputation, Stat Methods Med Res
Network, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies
Pan, Peto, Henao-Restrepo, Repurposed antiviral drugs for COVID-19 -interim WHO Solidarity trial results, N Engl J Med
Pardo, Shukla, Chamarthi, The journey of remdesivir: from Ebola to COVID-19, DIC
Parr, Time to reassess Tocilizumab's role in COVID-19 pneumonia, JAMA Intern Med
Petrosillo, Viceconte, Ergonul, COVID-19, SARS and MERS: are they closely related? Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases, Clin Microbiol Infect
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, J Virus Eradic
Pishgar, Greifer, Leyrat, MatchThem: matching and weighting after multiple imputation
Rafi, Bhattacharje, Al-Khafaji, Combination of QSAR, molecular docking, molecular dynamic simulation and MM-PBSA: analogues of lopinavir and favipiravir as potential drug candidates against COVID-19, J Biomol Struct Dyn
Rattanaumpawan, Jirajariyavej, Lerdlamyong, Realworld experience with favipiravir for treatment of COVID-19 in Thailand: results from a multi-center observational study, medRxiv
Scheike, Zhang, Analyzing competing risk data using the R timereg package, J Stat Soft
Sissoko, Laouenan, Folkesson, Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med
Sterne, White, Carlin, Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls, BMJ
Therneau, A package for survival analysis in R
Vaira, Deiana, Fois, Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases, Head Neck
Vaira, Hopkins, Salzano, Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study, Head Neck
Van Buuren, Groothuis-Oudshoorn, mice: Multivariate imputation by chained equations in R, J Stat Soft
Vassar, Holzmann, The retrospective chart review: important methodological considerations, J Educ Eval Health Prof
Wang, Chen, Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment, Eur J Pharmacol
Wooding, Bach, Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks, Clin Microbiol Infect
DOI record:
{
"DOI": "10.1080/03007995.2021.1920900",
"ISSN": [
"0300-7995",
"1473-4877"
],
"URL": "http://dx.doi.org/10.1080/03007995.2021.1920900",
"alternative-id": [
"10.1080/03007995.2021.1920900"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=icmo20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=icmo20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-02-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2021-04-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2021-04-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2021-05-19"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2091-1376",
"affiliation": [
{
"name": "Center for Health Outcomes and PharmacoEconomic Research, University of Arizona, Tucson, AZ, USA"
},
{
"name": "Department of Clinical Pharmacy, Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Alamer",
"given": "Ahmad",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Alrashed",
"given": "Ahmed A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, King Saud Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Alfaifi",
"given": "Mashael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4719-2655",
"affiliation": [
{
"name": "Research Center, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Alosaimi",
"given": "Bandar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia"
}
],
"family": "AlHassar",
"given": "Fatimah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, College of Pharmacy, Almaarefa University, Riyadh, Saudi Arabia"
}
],
"family": "Almutairi",
"given": "Malak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Howaidi",
"given": "Jude",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy, Shaqra University, Riyadh, Saudi Arabia"
}
],
"family": "Almutairi",
"given": "Wedad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, King Saud Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Mohzari",
"given": "Yahya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Sulaiman",
"given": "Tarek",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4730-9086",
"affiliation": [
{
"name": "Ministry of Health, Deputyship of Therapeutic Affairs, Riyadh, Saudi Arabia"
},
{
"name": "Alfaisal University, Colleges of Pharmacy and Medicine, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Al-jedai",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Services Administration, King Saud Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Alajami",
"given": "Hamdan N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Alkharji",
"given": "Fatima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Neurology Department, King Fahad Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Alsaeed",
"given": "Ali",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4684-2978",
"affiliation": [
{
"name": "Department of Infectious Diseases, King Saud Medical City, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Alali",
"given": "Alaa H.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4063-2857",
"affiliation": [
{
"name": "Department of Infectious Diseases, King Saud Medical City, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Baredhwan",
"given": "Abdullah A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0490-4421",
"affiliation": [
{
"name": "Center for Health Outcomes and PharmacoEconomic Research, University of Arizona, Tucson, AZ, USA"
},
{
"name": "Department of Pharmacy Practice and Science, College of Pharmacy, University of Arizona, Tucson, AZ, USA"
}
],
"authenticated-orcid": false,
"family": "Abraham",
"given": "Ivo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3727-7543",
"affiliation": [
{
"name": "Department of Pharmacy Practice, College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Almulhim",
"given": "Abdulaziz S.",
"sequence": "additional"
}
],
"container-title": [
"Current Medical Research and Opinion"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2021,
4,
23
]
],
"date-time": "2021-04-23T12:44:04Z",
"timestamp": 1619181844000
},
"deposited": {
"date-parts": [
[
2021,
8,
26
]
],
"date-time": "2021-08-26T08:15:17Z",
"timestamp": 1629965717000
},
"indexed": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T23:51:33Z",
"timestamp": 1640217093093
},
"is-referenced-by-count": 3,
"issn-type": [
{
"type": "print",
"value": "0300-7995"
},
{
"type": "electronic",
"value": "1473-4877"
}
],
"issue": "7",
"issued": {
"date-parts": [
[
2021,
5,
19
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2021,
7,
3
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/03007995.2021.1920900",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1085-1097",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2021,
5,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
19
]
]
},
"published-print": {
"date-parts": [
[
2021,
7,
3
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.cmi.2020.03.026",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1002/hed.26204",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1002/hed.26269",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.7573/dic.2020-4-14",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "CIT0006"
},
{
"DOI": "10.1038/d41573-020-00025-z",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"key": "CIT0013",
"unstructured": "Saudi Arabia Ministry of Health (MOH). Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19. [cited 2020 Sep 2]. Available from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf."
},
{
"key": "CIT0014",
"unstructured": "Equator Network. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. 2020."
},
{
"DOI": "10.3352/jeehp.2013.10.12",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"key": "CIT0017",
"unstructured": "Saudi Arabia Ministry of Health (MOH). Coronavirus Disease COVID-19 Guidelines, v1.3. [cited 2020 Sep 2]. Available from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/Coronavirus-Disease-2019-Guidelines-v1.2.pdf."
},
{
"key": "CIT0018",
"unstructured": "World Health Organization. Criteria for releasing COVID-19 patients from isolation. [cited 2020 Mar 22]. Available from: https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation."
},
{
"key": "CIT0019",
"unstructured": "U.S Department of Health and Human Services & National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. [cited 2020 Sep 2]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf."
},
{
"key": "CIT0020",
"unstructured": "Therneau TM. A package for survival analysis in R. 2020. [cited 2020 Aug 1]. Available from: https://CRAN.R-project.org/package=survival."
},
{
"DOI": "10.18637/jss.v038.i02",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.1136/bmj.b2393",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"author": "van Buuren S",
"issue": "3",
"journal-title": "J Stat Soft",
"key": "CIT0023",
"volume": "1",
"year": "2011"
},
{
"DOI": "10.1002/9781119536604.ch10",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"author": "Pishgar F",
"journal-title": "arXiv e-Prints 2020:arXiv:2009.11772",
"key": "CIT0025"
},
{
"DOI": "10.1177/0962280212445945",
"doi-asserted-by": "publisher",
"key": "CIT0026"
},
{
"DOI": "10.18637/jss.v009.i08",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"author": "Pan H",
"journal-title": "N Engl J Med",
"key": "CIT0028"
},
{
"key": "CIT0029",
"unstructured": "U.S Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. [cited 2020 Aug 1]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19."
},
{
"DOI": "10.1016/j.cmi.2020.08.005",
"doi-asserted-by": "publisher",
"key": "CIT0030"
},
{
"DOI": "10.1001/jamainternmed.2020.6557",
"doi-asserted-by": "publisher",
"key": "CIT0031"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "CIT0032"
},
{
"author": "Rafi MO",
"first-page": "1",
"journal-title": "J Biomol Struct Dyn",
"key": "CIT0033",
"volume": "30",
"year": "2020"
},
{
"key": "CIT0034",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. [cited 2020 Aug 1]. Available from: https://www.covid19treatmentguidelines.nih.gov."
},
{
"key": "CIT0035",
"unstructured": "Brigham and women's. Brigham and women's hospital inpatient COVID 19 infectious diseases treatment guidelines. [cited 2020 Aug 1]. Available from: https://covidprotocols.org/en/chapters/treatments/."
},
{
"DOI": "10.1016/j.ejphar.2020.173634",
"doi-asserted-by": "publisher",
"key": "CIT0036"
},
{
"author": "Rattanaumpawan P",
"journal-title": "medRxiv",
"key": "CIT0037",
"year": "2020"
},
{
"author": "Irie K",
"first-page": "880",
"issue": "5",
"journal-title": "Clin Transl Sci",
"key": "CIT0038",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.105.594952",
"doi-asserted-by": "publisher",
"key": "CIT0039"
},
{
"DOI": "10.1038/sj.bjc.6601119",
"doi-asserted-by": "publisher",
"key": "CIT0040"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"score": 1,
"short-container-title": [
"Current Medical Research and Opinion"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "37"
}