Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study
et al., Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129, Feb 2023
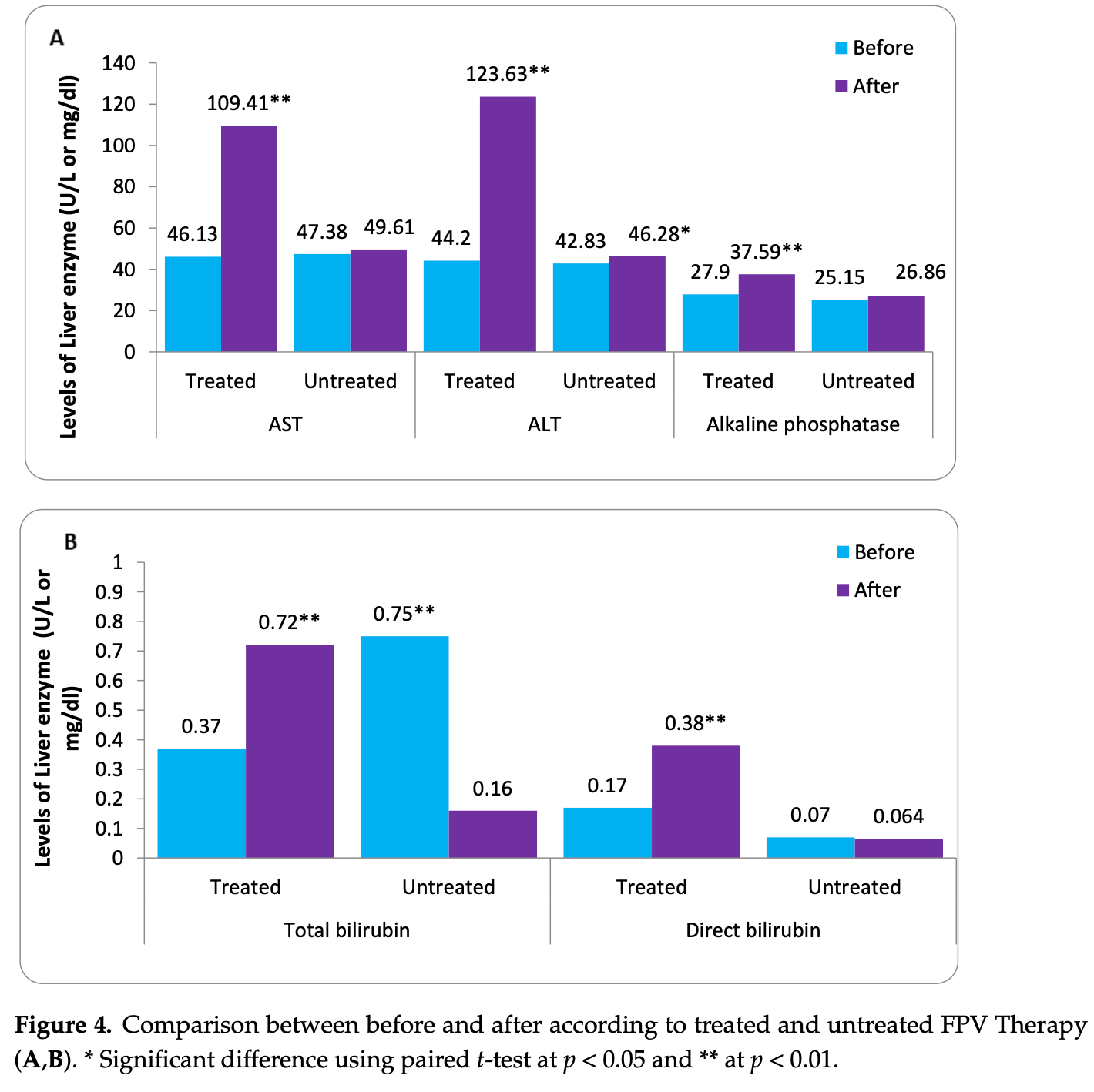
Retrospective 362 COVID-19 patients showing significant liver injury in favipiravir-treated patients compared to untreated controls.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Almutairi et al., 20 Feb 2023, retrospective, Saudi Arabia, peer-reviewed, mean age 51.3, 8 authors, study period May 2020 - August 2020.
Contact: mzreadi@uqu.edu.sa (corresponding author), syeid@uqu.edu.sa.
Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study
Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129
1) Background: Favipiravir (FVP) is a new antiviral drug used to treat COVID-19. It has been authorized to be used in the kingdom of Saudi Arabia in the treatment of COVID-19. The mechanism of action of FVP is working as a specific inhibitor for the RNA-dependent RNA polymerase of the RNA chain virus. FVP has the potential to be hepatotoxic because of the structure similarity with pyrazinamide. This retrospective study aimed to determine the prevalence of liver injury in FVP-treated COVID-19 patients in General East Jeddah Hospital, Saudi Arabia, during the COVID-19 pandemic. (2) Methods: A total of 6000 patients infected with COVID-19 and treated at the East Jeddah Hospital were included, with a sample size of 362 patients. The participants ranged from 18 to 70 years of age, both males and females, with normal hepatic and renal function and had a confirmed diagnosis of COVID-19 infection. Patients who had gouty arthritis, hepatic and renal dysfunction, dead patients, pregnant women, and breastfeeding mothers were all excluded from this study. A retrospective cohort study compared two groups of patients treated with and without FVP and who followed the Saudi Ministry of Health protocol to manage COVID-19 infection. (3) Results: An adverse effect of FVP on the liver was found that ranged from mild to severe. Stopping treatment with FVP was associated with an observed important increase in the levels of liver enzymes AST (p < 0.001), ALT (p < 0.001), alkaline phosphatase (p < 0.03), total bilirubin (p < 0.001), and direct bilirubin (p < 0.001) in the treated compared with the untreated group. ( 4 ) Conclusion: This study showed a significant difference between the treated and the untreated groups with FVP in liver injury. FVP influences the liver, increasing the blood levels of the liver function parameters.
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
References
Agrawal, Raju, Udwadia, Favipiravir, A new and emerging antiviral option in COVID-19, Med. J. Armed Forces India, doi:10.1016/j.mjafi.2020.08.004
Al-Shammari, Shahadha, The effect of Favipiravir on liver enzyme among patients with mild to moderate COVID-19 infection: A prospective cohort study, J. Popul. Ther. Clin. Pharmacol
Almoosa, Saad, Qara, Mustafa, Mansour et al., Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study, J. Infect. Public Health, doi:10.1016/j.jiph.2021.08.022
Alotaibi, Ali, Bakhshwin, Alatawi, Alotaibi et al., Effectiveness and Safety of Favipiravir Compared to Hydroxychloroquine for Management of Covid-19: A Retrospective Study, Int. J. Gen. Med, doi:10.2147/IJGM.S329881
Ara Perveen, Nasir, Murshed, Naznin, Ahmed, Remdesivir and Favipiravir Changes Hepato-Renal Profile in COVID-19 patients: A Cross Sectional Observation in Bangladesh, Int. J. Med. Sci. Clin. Invent, doi:10.18535/ijmsci/v8i01.03
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin. Pharmacol. Ther, doi:10.1002/cpt.1909
Carleton, Haynes, Histological Technique
Cascella, Rajnik, Aleem, Dulebohn, Di Napoli, Features, evaluation, and treatment of coronavirus (COVID-19)
Cicho Ż-Lach, Michalak, Liver injury in the era of COVID-19, World J Gastroenterol, doi:10.3748/wjg.v27.i5.377
Cieslak, Baur, Verheij, Bennink, Van Gulik, Liver function declines with increased age, Hpb
Da Rosa Mesquita, Francelino Silva Junior, Santos Santana, Farias De Oliveira, Campos Alcântara et al., Clinical manifestations of COVID-19 in the general population: Systematic review, Wien. Klin. Wochenschr, doi:10.1007/s00508-020-01760-4
Du, Chen, Favipiravir, Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin. Pharmacol. Ther, doi:10.1002/cpt.1844
Esakandari, Nabi-Afjadi, Fakkari-Afjadi, Farahmandian, Miresmaeili et al., A comprehensive review of COVID-19 characteristics, Biol. Proced. Online, doi:10.1186/s12575-020-00128-2
Furuta, Gowen, Takahashi, Shiraki, Smee et al., T-705), a novel viral RNA polymerase inhibitor, Antivir. Res, doi:10.1016/j.antiviral.2013.09.015
Ge, Yang, -M.; Xia, Fu, Zhang, Possible aerosol transmission of COVID-19 and special precautions in dentistry, J. Zhejiang Univ.-SCIENCE B, doi:10.1631/jzus.B2010010
Ghasemnejad-Berenji, Pashapour, Favipiravir and COVID-19: A simplified summary, Drug Res, doi:10.1055/a-1296-7935
Hashemian, Farhadi, Velayati, A review on favipiravir: The properties, function, and usefulness to treat COVID-19, Expert Rev. Anti-Infect. Ther, doi:10.1080/14787210.2021.1866545
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Sci. Rep, doi:10.1038/s41598-021-90551-6
Kaur, Charan, Dutta, Sharma, Bhardwaj et al., Favipiravir use in COVID-19: Analysis of suspected adverse drug events reported in the WHO database, Infect. Drug Resist, doi:10.2147/IDR.S287934
Kawasuji, Tsuji, Ogami, Takegoshi, Kaneda et al., Association between high serum favipiravir concentrations and drug-induced liver injury, medRxiv, doi:10.1101/2021.05.03.21256437
Kumar, Kulkarni, Sharma, Rao, Reddy, Favipiravir-induced liver injury in patients with coronavirus disease 2019, J. Clin. Transl. Hepatol, doi:10.14218/JCTH.2021.00011
Lala, Goyal, Minter, Liver function tests
Lam, Lombardi, Ouanounou, COVID-19: A review of the proposed pharmacological treatments, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173451
Lotfi, Hamblin, Rezaei, COVID-19: Transmission, prevention, and potential therapeutic opportunities, Clin. Chim. Acta, doi:10.1016/j.cca.2020.05.044
Lv, Huang, Account of Deep Learning-Based Ultrasonic Image Feature in the Diagnosis of Severe Sepsis Complicated with Acute Kidney Injury, Comput. Math Methods Med, doi:10.1155/2022/8158634
Marc, Moldovan, Hoza, Restea, Sachelarie et al., Evaluation of hepatic biochemical parameters during antiviral treatment in COVID-19 patients, Biology, doi:10.3390/biology11010013
Morgan, Samuel, Vandeputte, Hayes, Plevris, SARS-CoV-2 infection and the liver, Pathogens, doi:10.3390/pathogens9060430
Nasir, Perveen, Murshed, Nazneen, Talha, Survival and biomarkers of COVID-19 patients treated with remdesivir and favipiravir in ICU during the peak of pandemic: A single center study in Bangladesh, J. Pharm. Res. Int, doi:10.9734/jpri/2020/v32i4531088
Ou, Lee, Tsai, Tseng, Chu et al., Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors, J. Pers. Med, doi:10.3390/jpm12010043
Pascarella, Strumia, Piliego, Bruno, Del Buono et al., COVID-19 diagnosis and management: A comprehensive review, J. Intern. Med, doi:10.1111/joim.13091
Saudi, Creatinine, Lfts, Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19. Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection
Sodeifian, Seyedalhosseini, Kian, Eftekhari, Najari et al., Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review, Front. Med, doi:10.3389/fmed.2021.731436
Sofi, Hamid, Bhat, SARS-CoV-2: A critical review of its history, pathogenesis, transmission, diagnosis and treatment, Biosaf. Health, doi:10.1016/j.bsheal.2020.11.002
Suh, Drug-induced liver injury, Yeungnam Univ. J. Med, doi:10.12701/yujm.2019.00297
Tajiri, Shimizu, Liver physiology and liver diseases in the elderly, World J. Gastroenterol, doi:10.3748/wjg.v19.i46.8459
Wu, Wang, Kuo, Shannar, Peter et al., An Update on Current Therapeutic Drugs Treating COVID-19, Curr Pharm. Rep, doi:10.1007/s40495-020-00216-7
Yamazaki, Suzuki, Sayama, Nakada, Igari et al., Suspected cholestatic liver injury induced by favipiravir in a patient with COVID-19, J. Infect. Chemother, doi:10.1016/j.jiac.2020.12.021
Yang, Zheng, Fan, Etiology and management of liver injury in patients with COVID-19, World J. Gastroenterol, doi:10.3748/wjg.v26.i32.4753
Zhang, Shi, Wang, Liver injury in COVID-19: Management and challenges, Lancet Gastroenterol. Hepatol, doi:10.1016/S2468-1253(20)30057-1
Zhao, Zhong, Mechanism of action of favipiravir against SARS-CoV-2: Mutagenesis or chain termination?, Innov, doi:10.1016/j.xinn.2021.100165
DOI record:
{
"DOI": "10.3390/tropicalmed8020129",
"ISSN": [
"2414-6366"
],
"URL": "http://dx.doi.org/10.3390/tropicalmed8020129",
"abstract": "<jats:p>(1) Background: Favipiravir (FVP) is a new antiviral drug used to treat COVID-19. It has been authorized to be used in the kingdom of Saudi Arabia in the treatment of COVID-19. The mechanism of action of FVP is working as a specific inhibitor for the RNA-dependent RNA polymerase of the RNA chain virus. FVP has the potential to be hepatotoxic because of the structure similarity with pyrazinamide. This retrospective study aimed to determine the prevalence of liver injury in FVP-treated COVID-19 patients in General East Jeddah Hospital, Saudi Arabia, during the COVID-19 pandemic. (2) Methods: A total of 6000 patients infected with COVID-19 and treated at the East Jeddah Hospital were included, with a sample size of 362 patients. The participants ranged from 18 to 70 years of age, both males and females, with normal hepatic and renal function and had a confirmed diagnosis of COVID-19 infection. Patients who had gouty arthritis, hepatic and renal dysfunction, dead patients, pregnant women, and breastfeeding mothers were all excluded from this study. A retrospective cohort study compared two groups of patients treated with and without FVP and who followed the Saudi Ministry of Health protocol to manage COVID-19 infection. (3) Results: An adverse effect of FVP on the liver was found that ranged from mild to severe. Stopping treatment with FVP was associated with an observed important increase in the levels of liver enzymes AST (p < 0.001), ALT (p < 0.001), alkaline phosphatase (p < 0.03), total bilirubin (p < 0.001), and direct bilirubin (p < 0.001) in the treated compared with the untreated group. (4) Conclusion: This study showed a significant difference between the treated and the untreated groups with FVP in liver injury. FVP influences the liver, increasing the blood levels of the liver function parameters.</jats:p>",
"alternative-id": [
"tropicalmed8020129"
],
"author": [
{
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Saudi Toxicology Society, Umm Al-Qura University, Makkah 24381, Saudi Arabia"
},
{
"name": "Clinical Pharmacy, General East Jeddah Hospital, Jeddah 22253, Saudi Arabia"
}
],
"family": "Almutairi",
"given": "Amal Oweid",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-8398-5412",
"affiliation": [
{
"name": "Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Biochemistry Department, Faculty of Pharmacy, Al-Azhar University, Assuit 71524, Egypt"
}
],
"authenticated-orcid": false,
"family": "El-Readi",
"given": "Mahmoud Zaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
}
],
"family": "Althubiti",
"given": "Mohammad",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5725-3522",
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Saudi Toxicology Society, Umm Al-Qura University, Makkah 24381, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Alhindi",
"given": "Yosra Zakariyya",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4490-889X",
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Saudi Toxicology Society, Umm Al-Qura University, Makkah 24381, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Ayoub",
"given": "Nahla",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7782-0574",
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Saudi Toxicology Society, Umm Al-Qura University, Makkah 24381, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Alzahrani",
"given": "Abdullah R.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1527-6515",
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
},
{
"name": "Saudi Toxicology Society, Umm Al-Qura University, Makkah 24381, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Al-Ghamdi",
"given": "Saeed S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Al Abdeyah, Makkah 24381, Saudi Arabia"
}
],
"family": "Eid",
"given": "Safaa Yehia",
"sequence": "additional"
}
],
"container-title": "Tropical Medicine and Infectious Disease",
"container-title-short": "TropicalMed",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T08:29:06Z",
"timestamp": 1676881746000
},
"deposited": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T10:51:07Z",
"timestamp": 1676890267000
},
"funder": [
{
"award": [
"22UQU4350123DSR02"
],
"name": "Deanship of Scientific Research at Umm Al-Qura University"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T20:46:35Z",
"timestamp": 1750797995737,
"version": "3.37.3"
},
"is-referenced-by-count": 4,
"issue": "2",
"issued": {
"date-parts": [
[
2023,
2,
20
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T00:00:00Z",
"timestamp": 1676851200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2414-6366/8/2/129/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "129",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
2,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
20
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.bsheal.2020.11.002",
"article-title": "SARS-CoV-2: A critical review of its history, pathogenesis, transmission, diagnosis and treatment",
"author": "Sofi",
"doi-asserted-by": "crossref",
"first-page": "217",
"journal-title": "Biosaf. Health",
"key": "ref_1",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1186/s12575-020-00128-2",
"article-title": "A comprehensive review of COVID-19 characteristics",
"author": "Esakandari",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Biol. Proced. Online",
"key": "ref_2",
"volume": "22",
"year": "2020"
},
{
"key": "ref_3",
"unstructured": "W.H.O. (2020, January 01). Coronavirus Disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1."
},
{
"DOI": "10.1111/joim.13091",
"article-title": "COVID-19 diagnosis and management: A comprehensive review",
"author": "Pascarella",
"doi-asserted-by": "crossref",
"first-page": "192",
"journal-title": "J. Intern. Med.",
"key": "ref_4",
"volume": "288",
"year": "2020"
},
{
"DOI": "10.1007/s00508-020-01760-4",
"article-title": "Clinical manifestations of COVID-19 in the general population: Systematic review",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "Wien. Klin. Wochenschr.",
"key": "ref_5",
"volume": "133",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2020.173451",
"article-title": "COVID-19: A review of the proposed pharmacological treatments",
"author": "Lam",
"doi-asserted-by": "crossref",
"first-page": "173451",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_6",
"volume": "886",
"year": "2020"
},
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"article-title": "Favipiravir: A new and emerging antiviral option in COVID-19",
"author": "Agrawal",
"doi-asserted-by": "crossref",
"first-page": "370",
"journal-title": "Med. J. Armed Forces India",
"key": "ref_7",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1007/s40495-020-00216-7",
"article-title": "An Update on Current Therapeutic Drugs Treating COVID-19",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Curr Pharm. Rep.",
"key": "ref_8",
"volume": "6",
"year": "2020"
},
{
"article-title": "Mechanism of action of favipiravir against SARS-CoV-2: Mutagenesis or chain termination?",
"author": "Zhao",
"first-page": "100165",
"journal-title": "Innov.",
"key": "ref_9",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"journal-title": "Antivir. Res.",
"key": "ref_10",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "242",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_11",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.2147/IDR.S287934",
"article-title": "Favipiravir use in COVID-19: Analysis of suspected adverse drug events reported in the WHO database",
"author": "Kaur",
"doi-asserted-by": "crossref",
"first-page": "4427",
"journal-title": "Infect. Drug Resist.",
"key": "ref_12",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2021.1866545",
"article-title": "A review on favipiravir: The properties, function, and usefulness to treat COVID-19. Expert Rev",
"author": "Hashemian",
"doi-asserted-by": "crossref",
"first-page": "1029",
"journal-title": "Anti-Infect. Ther.",
"key": "ref_13",
"volume": "19",
"year": "2021"
},
{
"key": "ref_14",
"unstructured": "Saudi, C., Creatinine, C., and LFTs, C.X. (2020). Coronavirus Diseases 19 (COVID-19) Guidelines. July 31st, Version 2.1, Ministry of Health."
},
{
"DOI": "10.1155/2022/8158634",
"article-title": "Account of Deep Learning-Based Ultrasonic Image Feature in the Diagnosis of Severe Sepsis Complicated with Acute Kidney Injury",
"author": "Lv",
"doi-asserted-by": "crossref",
"first-page": "8158634",
"journal-title": "Comput. Math Methods Med.",
"key": "ref_15",
"volume": "2022",
"year": "2022"
},
{
"article-title": "Favipiravir-induced liver injury in patients with coronavirus disease 2019",
"author": "Kumar",
"first-page": "276",
"journal-title": "J. Clin. Transl. Hepatol.",
"key": "ref_16",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"first-page": "11022",
"journal-title": "Sci. Rep.",
"key": "ref_17",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.731436",
"article-title": "Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review",
"author": "Sodeifian",
"doi-asserted-by": "crossref",
"first-page": "1624",
"journal-title": "Front. Med.",
"key": "ref_18",
"volume": "8",
"year": "2021"
},
{
"key": "ref_19",
"unstructured": "Cascella, M., Rajnik, M., Aleem, A., Dulebohn, S.C., and Di Napoli, R. (2022). Statpearls [Internet], StatPearls Publishing."
},
{
"DOI": "10.1016/j.jiac.2020.12.021",
"article-title": "Suspected cholestatic liver injury induced by favipiravir in a patient with COVID-19",
"author": "Yamazaki",
"doi-asserted-by": "crossref",
"first-page": "390",
"journal-title": "J. Infect. Chemother.",
"key": "ref_20",
"volume": "27",
"year": "2021"
},
{
"article-title": "The effect of Favipiravir on liver enzyme among patients with mild to moderate COVID-19 infection: A prospective cohort study",
"author": "Shahadha",
"first-page": "46",
"journal-title": "J. Popul. Ther. Clin. Pharmacol.",
"key": "ref_21",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.3390/jpm12010043",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Ou, S.M., Lee, K.H., Tsai, M.T., Tseng, W.C., Chu, Y.C., and Tarng, D.C. (2022). Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors. J. Pers. Med., 12."
},
{
"DOI": "10.12701/yujm.2019.00297",
"article-title": "Drug-induced liver injury",
"author": "Suh",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Yeungnam Univ. J. Med.",
"key": "ref_23",
"volume": "37",
"year": "2020"
},
{
"key": "ref_24",
"unstructured": "Carleton, H.M., and Haynes, F. (1926). Histological Technique, Oxford University Press."
},
{
"DOI": "10.1631/jzus.B2010010",
"article-title": "Possible aerosol transmission of COVID-19 and special precautions in dentistry",
"author": "Ge",
"doi-asserted-by": "crossref",
"first-page": "361",
"journal-title": "J. Zhejiang Univ.-SCIENCE B",
"key": "ref_25",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3748/wjg.v27.i5.377",
"article-title": "Liver injury in the era of COVID-19",
"author": "Michalak",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "World J Gastroenterol.",
"key": "ref_26",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.cca.2020.05.044",
"article-title": "COVID-19: Transmission, prevention, and potential therapeutic opportunities",
"author": "Lotfi",
"doi-asserted-by": "crossref",
"first-page": "254",
"journal-title": "Clin. Chim. Acta",
"key": "ref_27",
"volume": "508",
"year": "2020"
},
{
"DOI": "10.1055/a-1296-7935",
"article-title": "Favipiravir and COVID-19: A simplified summary",
"author": "Pashapour",
"doi-asserted-by": "crossref",
"first-page": "166",
"journal-title": "Drug Res.",
"key": "ref_28",
"volume": "71",
"year": "2021"
},
{
"DOI": "10.9734/jpri/2020/v32i4531088",
"article-title": "Survival and biomarkers of COVID-19 patients treated with remdesivir and favipiravir in ICU during the peak of pandemic: A single center study in Bangladesh",
"author": "Nasir",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "J. Pharm. Res. Int.",
"key": "ref_29",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.3748/wjg.v26.i32.4753",
"article-title": "Etiology and management of liver injury in patients with COVID-19",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "4753",
"journal-title": "World J. Gastroenterol.",
"key": "ref_30",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2021.08.022",
"article-title": "Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study",
"author": "Almoosa",
"doi-asserted-by": "crossref",
"first-page": "1247",
"journal-title": "J. Infect. Public Health",
"key": "ref_31",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1101/2021.05.03.21256437",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Kawasuji, H., Tsuji, Y., Ogami, C., Takegoshi, Y., Kaneda, M., Murai, Y., Kimoto, K., Ueno, A., Miyajima, Y., and Fukui, Y.J.m. (2021). Association between high serum favipiravir concentrations and drug-induced liver injury. medRxiv."
},
{
"key": "ref_33",
"unstructured": "Lala, V., Goyal, A., and Minter, D.A. (2021). StatPearls [Internet], StatPearls Publishing."
},
{
"DOI": "10.3390/biology11010013",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Marc, F., Moldovan, C., Hoza, A., Restea, P., Sachelarie, L., Romila, L.E., Suteu, C., and Farcas, D.M. (2021). Evaluation of hepatic biochemical parameters during antiviral treatment in COVID-19 patients. Biology, 11."
},
{
"DOI": "10.2147/IJGM.S329881",
"article-title": "Effectiveness and Safety of Favipiravir Compared to Hydroxychloroquine for Management of Covid-19: A Retrospective Study",
"author": "Alotaibi",
"doi-asserted-by": "crossref",
"first-page": "5597",
"journal-title": "Int. J. Gen. Med.",
"key": "ref_35",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.18535/ijmsci/v8i01.03",
"article-title": "Remdesivir and Favipiravir Changes Hepato-Renal Profile in COVID-19 patients: A Cross Sectional Observation in Bangladesh",
"author": "Nasir",
"doi-asserted-by": "crossref",
"first-page": "5196",
"journal-title": "Int. J. Med. Sci. Clin. Invent.",
"key": "ref_36",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_37",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.3748/wjg.v19.i46.8459",
"article-title": "Liver physiology and liver diseases in the elderly",
"author": "Tajiri",
"doi-asserted-by": "crossref",
"first-page": "8459",
"journal-title": "World J. Gastroenterol.",
"key": "ref_38",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.3390/pathogens9060430",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Morgan, K., Samuel, K., Vandeputte, M., Hayes, P.C., and Plevris, J.N.J.P. (2020). SARS-CoV-2 infection and the liver. Pathogens, 9."
},
{
"DOI": "10.1016/S2468-1253(20)30057-1",
"article-title": "Liver injury in COVID-19: Management and challenges",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "428",
"journal-title": "Lancet Gastroenterol. Hepatol.",
"key": "ref_40",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.hpb.2016.05.011",
"article-title": "Liver function declines with increased age",
"author": "Cieslak",
"doi-asserted-by": "crossref",
"first-page": "691",
"journal-title": "Hpb",
"key": "ref_41",
"volume": "18",
"year": "2016"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2414-6366/8/2/129"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study",
"type": "journal-article",
"volume": "8"
}