Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats
et al., Journal of Advanced Veterinary Research, 13:10, Dec 2023
Animal study showing organ toxicity induced by favipiravir in rats. Histopathological and clinical results showed that favipiravir caused lesions in the liver, kidney and lung as well as increased liver enzymes, kidney function markers, oxidative stress, and inflammation. The effects were dose-dependent, with higher doses causing more severe changes.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
El-Fetouh et al., 1 Dec 2023, peer-reviewed, 4 authors.
Experimental Studies on Some Drugs Used in
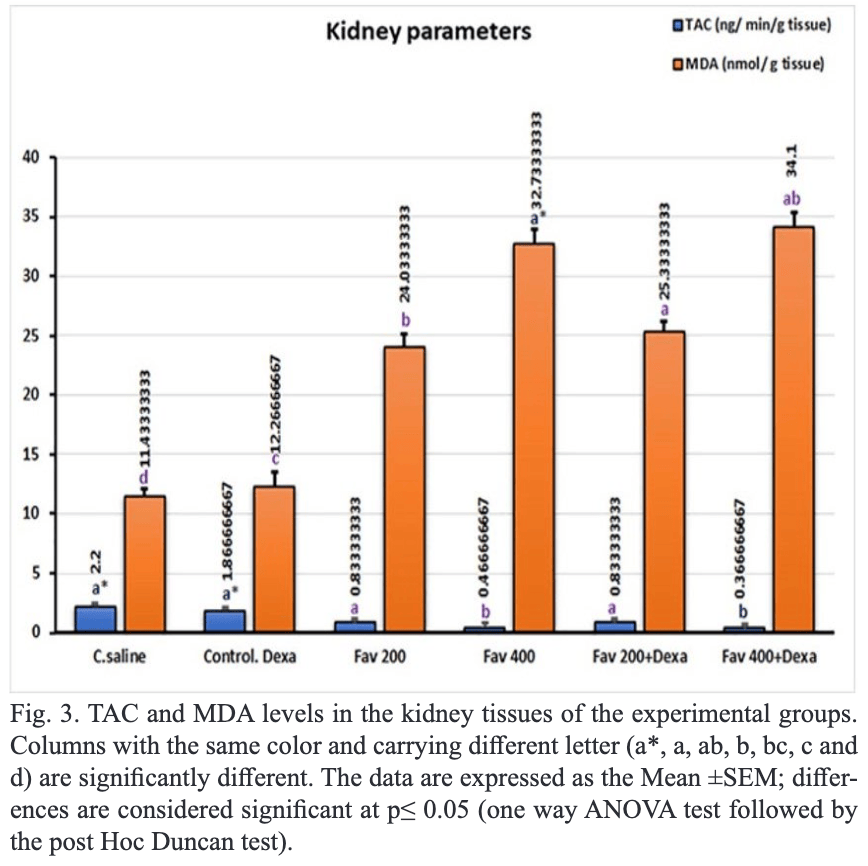
in Albino Rats In this study, the side effects of some anti-covid-19 drugs (Favipiravir and Dexamethasone) were evaluated through the pathological, and clinicopathological changes in the tissues of rats. 30 rats were divided into 6 groups: Gp1-control; Gp2 received 0.54 mg/kg dexamethasone; Gp3 received 200 mg/kg favipiravir; Gp4 received 400 mg/kg favipiravir; Gp5 received 200 mg/kg favipiravir + 0.54 mg/kg dexamethasone, and Gp6 received 400 mg/kg favipiravir + 0.54 mg/kg dexamethasone. Histopathological and clinical results showed that both favipiravir and dexamethasone-induced lesions in the liver, kidney, and lung as well as increased liver functions (alanine transaminase, aspartate aminotransferase, and C-reactive protein) and kidney functions (urea and creatinine). Also increased oxidative stress parameters such as malondialdehyde and decreased antioxidants in liver, and kidney tissues. Gene expression in splenic tissues showed an increase in NF-kb, IL6, and TNF when animals were exposed to 400 mg/kg favipiravir. While these genes (NF-kb, IL6, and TNF) decreased when animals received a combination of favipiravir with dexamethasone. In gp3, hydropic degeneration was noted in both the kidney and liver. In Gp4, necrotic changes in the liver, and vacuolation of the renal glomerular tufts were observed. In Gp5, the necrotic hepatic tissues were infiltrated with mononuclear cells, and necrosis and inflammation in renal tubules in the kidney were shown. In gp6, leukocytic infiltration was noted in both the kidney and liver. In conclusion, the anti-Covid-19 drugs could induce pathological changes in the internal organs of the rat.
CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
References
Akbal-Dagistan, Sevim, Sen, Basarir, Culha et al., Pulmonary delivery of favipiravir in rats reaches high local concentrations without causing oxidative lung injury or systemic side effects, Pharmaceutics
Atçali, Yakut, Çağlayan, Ulucan, Kara, Effects of favipiravir on hematologic parameters and bone marrow in the rats, J. Exper. Clin. Med
Balcı, Çöllüoğlu, Yavuzer, Bulut, Altındağ et al., Effect of low and high dose of favipiravir on ovarian and reproductive function in female rats: Biochemical and histopathological evaluation, Gen. Physiol. Biophys
Baranovich, Wong, Armstrong, Marjuki, Webby et al., T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro, J. Virol
Bilici, Altuner, Suleyman, Bulut, Sarigul et al., Favipiravir-induced inflammatory and hydropic degenerative liver injury in rats
Ciotti, Ciccozzi, Terrinoni, Jiang, Wang et al., The COVID-19 pandemic, Crit. Critical Rev. Clin. Lab. Sci
Doğan, Kaya, Demirel, Başeğmez, Şahin et al., The effect of vitamin C supplementation on favipiravir-induced oxidative stress and proinflammatory damage in livers and kidneys of rats, Immunopharmacol. Immunotoxicol
Driouich, Cochin, Lingas, Moureau, Touret et al., Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nature Commun
He, Deng, Li, Coronavirus disease 2019: What we know?, J. Med. Virol
Kara, Yakut, Caglayan, Atçalı, Ulucan et al., Evaluation of the toxicological effects of favipiravir (T-705) on liver and kidney in rats: biochemical and histopathological approach, Drug Chem. Toxicol
Kaur, Charan, Dutta, Sharma, Bhardwaj et al., Favipiravir use in COVID-19: analysis of suspected adverse drug events reported in the WHO database, Infect. Drug Resist
Kumar, Kulkarni, Sharma, Rao, Reddy, Favipiravir-induced liver injury in patients with coronavirus disease 2019, J. Clin. Transl. Hepatol
Li, Liu, Yu, Tang, Tang, Coronavirus disease 2019 (COVID-19): current status and future perspectives, International J. Antimicrob. Agents
Livak, Schmittgen, Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 -ΔΔCT Method, Methods
Marra, Smolders, El-Sherif, Boyle, Davidson et al., Recommendations for dosing of repurposed COVID-19 medications in patients with renal and hepatic impairment, Drugs
Ozbas, Kayan, Yakarisik, Dulger, Ayvaz et al., Role of Favipiravir on the Hematologic parameters in patients with COVID-19 infection: Favipiravir Related Hematologic parameters, Med. Sci. Discov
Samson, Pranith, Kumar, Prasobh, A review on the use of favipiravir in treatment of covid .91, World J. Pharm. Res
Siniscalco, Giordano, Galderisi, Luongo, De Novellis et al., Integrative Neuroscience Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice, Front. Integr. Neurosci
Tarighi, Eftekhari, Chizari, Sabernavaei, Jafari et al., A review of potential suggested drugs for coronavirus disease (COVID-19) treatment, Europ. J. Pharmacol
Tastemel Ozturk, Baltu, Kurt Sukur, Ozsurekci, Gucer et al., Acute kidney injury in a patient with COVID-19: Questions, Ped. Nephrol
Yamazaki, Suzuki, Sayama, Nakada, Igari et al., Suspected cholestatic liver injury induced by favipiravir in a patient with COVID-19, J. Infect. Chemother
Yuki, Fujiogi, Koutsogiannaki, COVID-19 pathophysiology: A review, Clin. Immunol