Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans
et al., Heliyon, doi:10.1016/j.heliyon.2024.e35331, Jul 2024
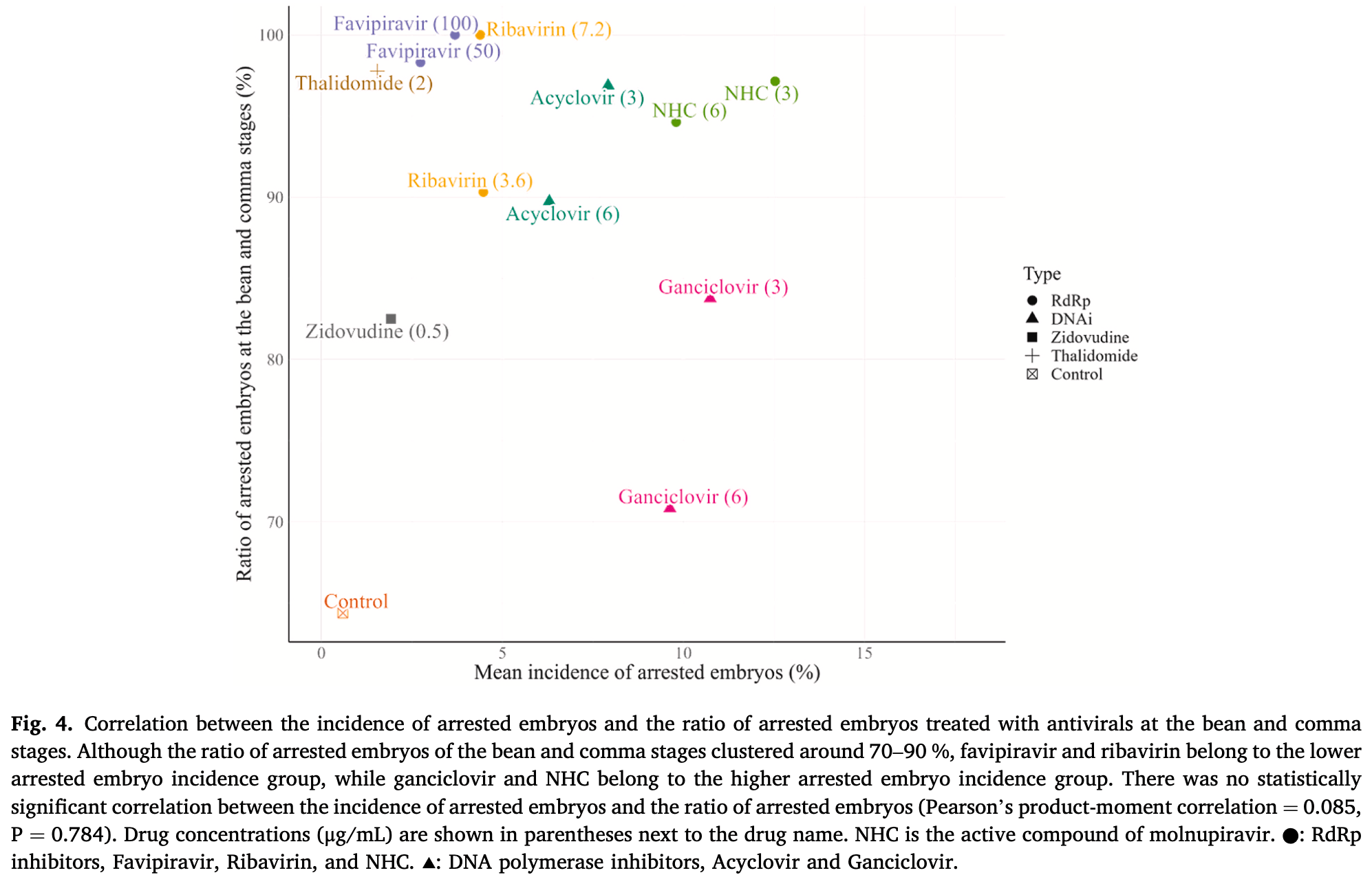
Reproductive toxicity analysis of antiviral drugs in C. elegans, showing increased incidence of arrested embryos with molnupiravir, favipiravir, ribavirin, acyclovir, ganciclovir, zidovudine, and thalidomide compared to controls. Authors hypothesize that RNA-dependent RNA polymerase (RdRp) inhibitors impair RNA interference through RdRp expressed during embryogenesis, causing embryo-fetal toxicity. Favipiravir was less toxic than molnupiravir. While findings align with reproductive toxicity observed in other studies, authors note that human DNA polymerase specificity and metabolism may differ. Authors suggest the relatively higher toxicity of many drugs compared to favipiravir may be due to favipiravir's high specificity for RNA-dependent RNA polymerase (RdRp) inhibition without affecting cellular DNA synthesis. Authors conclude that the C. elegans screening system can be a useful tool for initial reproductive toxicity screening of antiviral compounds before proceeding to animal studies.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Study covers molnupiravir and favipiravir.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Shiraki et al., 26 Jul 2024, Japan, peer-reviewed, 5 authors.
Contact: shirakitoyama@gmail.com, japan.shirakitoyama@gmail.com.
Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans
Heliyon, doi:10.1016/j.heliyon.2024.e35331
Reproductive toxicity is one of the major concerns in drug development. Thus, we have developed its screening system using Caenorhabditis elegans, which has a life cycle of three days and similar coding genes as humans. Antiviral nucleoside analogs used for acute infections are known to cause reproductive toxicity, contraindicated for pregnant women, and are used for comparing their reproductive toxicity in C. elegans and experimental animals. None of the drug treatments affected the number of offspring and the concentrations without toxicity to nematodes were consistent with no cytotoxicity or toxicity in experimental animals or humans. Favipiravir, ribavirin, molnupiravir (NHC), acyclovir, ganciclovir, zidovudine, and thalidomide significantly increased the incidence of arrested embryos but amenamevir, letermovir, and guanosine did not. RNA-dependent RNA polymerase (RdRp) inhibitors, in the order of favipiravir, ribavirin, and NHC increased the incidence of arrested embryos, possibly due to the specificity of favipiravir for RdRp and less cytotoxicity. RdRp inhibitors would impair RNA interference through RdRp expressed by telomerase reverse transcriptase during embryogenesis and cause embryo-fetal toxicity. The incidence of arrested embryos may be affected by differences in the substrate specificity of DNA polymerases and metabolism between C. elegans, animals, and humans. The concordance between the results of the screening system for reproductive toxicity of antivirals in C. elegans and those in experimental animals based on the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, reproductive toxicology confirms its appropriateness as a screening system for reproductive toxicity. Favipiravir and zidovudine were the least toxic to C. elegans among the antiviral drugs examined.
Administration period: 0-7 days gestation (until implantation). Summary on the 20th day of gestation to observe the endometrium and foetation. $, Human AUC was based on the following dose: favipiravir, 1600 mg × 2/day (loading dose for influenza patients); ribavirin, 400 mg × 2/day (dose for 60-80 kg patients); valacyclovir, 1000 mg × 3/day (dose for zoster patients). Statistically significant difference from the vehicle control: *, p < 0.05; **, p < 0.01. The table is provided with permission by Fujifilm Toyama Chemical Co., Ltd [17] . The authors obtained permission from Pharmacology & Therapeutics to reuse this table [77] .
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Altun, Herndon, Wolkow, WormAtlas
Asatsuma-Okumura, Ando, Simone, Yamamoto, Sato et al., p63 is a cereblon substrate involved in thalidomide teratogenicity, Nat. Chem. Biol
Awad, Thorlund, Hauser, Stimac, Mabrouk et al., Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials, Hepatology
Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell
Bernstein, Caudy, Hammond, Hannon, Role for a bidentate ribonuclease in the initiation step of RNA interference, Nature
Brenner, The genetics of Caenorhabditis elegans, Genetics
Byerly, Cassada, Russell, The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction, Dev. Biol
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Chahoud, Stahlmann, Bochert, Dillmann, Neubert, Gross-structural defects in rats after acyclovir application on day 10 of gestation, Arch. Toxicol
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus arbidol for COVID-19: a randomized clinical trial, medRxiv
Chono, Katsumata, Kontani, Kobayashi, Sudo et al., ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2, J. Antimicrob. Chemother
Co, Amenalief Tablets (200mg) CTD
Co, None, Amenalief Tablets
Collins, Bauer, The activity in vitro against herpes virus of 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine), a new antiviral agent, J. Antimicrob. Chemother
Culetto, Sattelle, A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes, Hum. Mol. Genet
D'amato, Loughnan, Flynn, Folkman, Thalidomide is an inhibitor of angiogenesis, Proc. Natl. Acad. Sci. U. S. A
Delang, Abdelnabi, Neyts, Favipiravir as a potential countermeasure against neglected and emerging RNA viruses, Antivir. Res
Dipaolo, Congenital malformation in strain A mice. Its experimental production by thalidomide, JAMA
Doi, Ishihara, Banno, Ando, Kondo et al., Favipiravir for symptomatic COVID-19: a nationwide observational cohort study, J. Infect. Chemother
Drucker, Uziel, Tohami, Shapiro, Radnay et al., Thalidomide down-regulates transcript levels of GC-rich promoter genes in multiple myeloma, Mol. Pharmacol
Eldridge, Ephross, Heffner, Tennis, Stender et al., Monitoring pregnancy outcomes following prenatal drug exposure through prospective pregnancy registries and passive surveillance: a pharmaceutical company commitment, Prim. Care Update OB/GYNS
Elegans, Sequencing Consortium, Genome sequence of the nematode C. elegans: a platform for investigating biology, Science
Ertem, Guner, Incir, Kalkan, Gelal, The outcomes of favipiravir exposure in pregnancy: a case series, Arch. Gynecol. Obstet
Fujifim, AVIGAN Tablets 200mg package insert
Furman, Fyfe, St Clair, Weinhold, Rideout et al., Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase, Proc. Natl. Acad. Sci. U. S. A
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antivir. Res
Furuta, Takahashi, Fukuda, Kuno, Kamiyama et al., In vitro and in vivo activities of anti-influenza virus compound T-705, Antimicrob. Agents Chemother
Gao, Wang, Fan, Hu, Recent advances in the molecular mechanism of thalidomide teratogenicity, Biomed. Pharmacother
Genentech, Valcyte(r) F, Tablets, None
Ghimire, Mantziou, Moris, Arias, Human gastrulation: the embryo and its models, Dev. Biol
Glaxosmithkline, VALTREX Valacyclovir tablets
Greene, Ayers, Tucker, Miranda, Nonclinical toxicology studies with zidovudine: reproductive toxicity studies in rats and rabbits, Fund. Appl. Toxicol
Guo, Ghosh, Keyes, Peterson, Forman et al., The synthesis and anti-cytomegalovirus activity of piperidine-4carboxamides, Viruses
Hayashi, Hayashi, Tomoda, Phenoxazine derivatives inactivate human cytomegalovirus, herpes simplex virus-1, and herpes simplex virus-2 in vitro, J. Pharmacol. Sci
Hayflick, Moorhead, The serial cultivation of human diploid cell strains, Exp. Cell Res
Hobden, Kumar, Kaufman, Clement, Varnell et al., In vitro synergism of trifluorothymidine and ganciclovir against HSV-1, Invest. Ophthalmol. Vis. Sci
Hofer, Donnerer, Sator, Staufer, Scherzer et al., Seminal fluid ribavirin level and functional semen parameters in patients with chronic hepatitis C on antiviral combination therapy, J. Hepatol
Hunt, The C. elegans model in toxicity testing, J. Appl. Toxicol
Ich, None, Reprod. Toxicol
Ito, Ando, Suzuki, Ogura, Hotta et al., Identification of a primary target of thalidomide teratogenicity, Science
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial, Clin. Infect. Dis
Jacobs, Aarons, Bhagani, Buchanan, Cropley et al., Post-exposure prophylaxis against Ebola virus disease with experimental antiviral agents: a case-series of health-care workers, Lancet Infect. Dis
Kilham, Ferm, Congenital anomalies induced in hamster embryos with ribavirin, Science
Kim, Park, Hwang, Kwack, Comparative genomic analysis of the human and nematode Caenorhabditis elegans uncovers potential reproductive genes and disease associations in humans, Physiol. Genom
Klug, Lewandowski, Merker, Stahlmann, Wildi et al., In vitro and in vivo studies on the prenatal toxicity of five virustatic nucleoside analogues in comparison to aciclovir, Arch. Toxicol
Kochhar, Effects of exposure to high concentrations of ribavirin in developing embryos, Pediatr. Infect. Dis. J
Kong, Gao, Zhu, Zhang, Xue et al., Reproductive toxicity induced by nickel nanoparticles in Caenorhabditis elegans, Environ. Toxicol
Krilov, Safety issues related to the administration of ribavirin, Pediatr. Infect. Dis. J
Leung, Williams, Benedetto, Au, Helmcke et al., Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology, Toxicol. Sci
Machitani, Yasukawa, Nakashima, Furuichi, Masutomi, RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19, Cancer Sci
Maida, Yasukawa, Furuuchi, Lassmann, Possemato et al., An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA, Nature
Maida, Yasukawa, Masutomi, De novo RNA synthesis by RNA-dependent RNA polymerase activity of telomerase reverse transcriptase, Mol. Cell Biol
Merck, Co, Fact Sheet For Healthcare Providers: Emergency Use Authorization For Lagevrio™ (molnupiravir) Capsules
Merck, Co, PREVYMIS™ (letermovir) tablets
Nance, Lee, Goldstein, Gastrulation in C. elegans
Narayana, Souza, Seetharama Rao, Ribavirin-induced sperm shape abnormalities in Wistar rat, Mutat. Res
Olovnikov, A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon, J. Theor. Biol
Ozturk, Sozen, Demir, Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species, Mol. Hum. Reprod
Pasternak, Hviid, Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects, JAMA
Pecou, Moinard, Walschaerts, Pasquier, Daudin et al., Ribavirin and pegylated interferon treatment for hepatitis C was associated not only with semen alterations but also with sperm deoxyribonucleic acid fragmentation in humans, Fertil. Steril
Pescovitz, Absence of teratogenicity of oral ganciclovir used during early pregnancy in a liver transplant recipient, Transplantation
Pharmaceutical, None, Thaled® Capsule
Pmda, Report on the Deliberation Results (Avigan Tablet 200 Mg)
Salas-Huetos, James, Aston, Jenkins, Carrell et al., The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: a systematic review, Cells
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect. Dis. Ther
Shiraki, Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol. Ther
Shiraki, Helicase-primase inhibitor amenamevir for herpesvirus infection: towards practical application for treating herpes zoster, Drugs Today
Shiraki, None, Heliyon
Shiraki, None, Heliyon
Shiraki, None, Heliyon
Shiraki, Sato, Sakai, Matsumoto, Kaszynski et al., Antiviral therapy for COVID-19: derivation of optimal strategy based on past antiviral and favipiravir experiences, Pharmacol. Ther
Shiraki, Tan, Daikoku, Takemoto, Sato et al., Viral ribonucleotide reductase attenuates the anti-herpes activity of acyclovir in contrast to amenamevir, Antivir. Res
Shiraki, Yasumoto, Toyama, Fukuda, Amenamevir, a helicase-primase inhibitor, for the optimal treatment of herpes zoster, Viruses
Sidwell, Khare, Allen, Huffman, Witkowski et al., In vitro and in vivo effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) on types 1 and 3 parainfulenza virus infections, Chemotherapy
Sinclair, Jones, Miller, Greene, Kwo et al., The ribavirin pregnancy registry: an interim analysis of potential teratogenicity at the mid-point of enrollment, Drug Saf
Sissoko, Laouenan, Folkesson, M'lebing, Beavogui et al., None
Smee, Evans, Nicolaou, Tarbet, Day, Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds, Antivir. Res
Stahlmann, Klug, Foerster, Neubert, Significance of embryo culture methods for studying the prenatal toxicity of virustatic agents, Reprod. Toxicol
Stahlmann, Klug, Lewandowski, Bochert, Chahoud et al., Prenatal toxicity of acyclovir in rats, Arch. Toxicol
Suemori, Saijo, Yamanaka, Himeji, Kawamura et al., A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome, PLoS Neglected Trop. Dis
Tejeda-Benitez, Olivero, Verbel, Caenorhabditis elegans, a biological model for research in toxicology, Rev. Environ. Contam. Toxicol
Teo, Denny, Stirling, Thomas, Morseth et al., Effects of thalidomide on reproductive function and early embryonic development in male and female New Zealand white rabbits, Birth Defects Res B Dev Reprod Toxicol
Tirmikcioglu, Favipiravir exposure and pregnancy outcome of COVID-19 patients, Eur. J. Obstet. Gynecol. Reprod. Biol
Turner, Wong, Rai, Hartshorne, Telomere lengths in human oocytes, cleavage stage embryos and blastocysts, Mol. Hum. Reprod
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNAdependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int. J. Infect. Dis
Vastenhouw, Cao, Lipshitz, The maternal-to-zygotic transition revisited, Development
Wang, Fan, Salam, Horby, Hayden et al., Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection, J. Infect. Dis
Yamada, Noguchi, Komeno, Furuta, Nishizono, Efficacy of favipiravir (T-705) in rabies postexposure prophylaxis, J. Infect. Dis
Yamanaka, Murai, Saito, Abe, Tokunaga et al., Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF, EMBO J
Yuan, Lu, Yao, Zhao, Zhang et al., Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome, EBioMedicine
Zhou, Hill, Sarkar, Tse, Woodburn et al., beta-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells, J. Infect. Dis
DOI record:
{
"DOI": "10.1016/j.heliyon.2024.e35331",
"ISSN": [
"2405-8440"
],
"URL": "http://dx.doi.org/10.1016/j.heliyon.2024.e35331",
"alternative-id": [
"S240584402411362X"
],
"article-number": "e35331",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Heliyon"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.heliyon.2024.e35331"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5218-4249",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shiraki",
"given": "Kimiyasu",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mishima",
"given": "Mizuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Noriaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imoto",
"given": "Yasuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nishiwaki",
"given": "Kiyoji",
"sequence": "additional"
}
],
"container-title": "Heliyon",
"container-title-short": "Heliyon",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
27
]
],
"date-time": "2024-07-27T02:38:58Z",
"timestamp": 1722047938000
},
"deposited": {
"date-parts": [
[
2024,
8,
20
]
],
"date-time": "2024-08-20T01:28:15Z",
"timestamp": 1724117295000
},
"indexed": {
"date-parts": [
[
2024,
8,
20
]
],
"date-time": "2024-08-20T02:10:16Z",
"timestamp": 1724119816286
},
"is-referenced-by-count": 0,
"issue": "15",
"issued": {
"date-parts": [
[
2024,
8
]
]
},
"journal-issue": {
"issue": "15",
"published-print": {
"date-parts": [
[
2024,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
26
]
],
"date-time": "2024-07-26T00:00:00Z",
"timestamp": 1721952000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S240584402411362X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S240584402411362X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e35331",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
8
]
]
},
"published-print": {
"date-parts": [
[
2024,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "AVIGAN Tablets 200mg package insert",
"author": "Fujifim",
"key": "10.1016/j.heliyon.2024.e35331_bib1",
"series-title": "Fujifim ToyamaChemical Co. Ltd.",
"year": "2019"
},
{
"key": "10.1016/j.heliyon.2024.e35331_bib3",
"series-title": "Fact Sheet For Healthcare Providers: Emergency Use Authorization For Lagevrio™ (molnupiravir) Capsules",
"year": "2023"
},
{
"journal-title": "Reprod. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib4",
"year": "2020"
},
{
"DOI": "10.1126/science.282.5396.2012",
"article-title": "Sequencing Consortium, Genome sequence of the nematode C. elegans: a platform for investigating biology",
"author": "elegans",
"doi-asserted-by": "crossref",
"first-page": "2012",
"journal-title": "Science",
"key": "10.1016/j.heliyon.2024.e35331_bib5",
"volume": "282",
"year": "1998"
},
{
"DOI": "10.1038/nature03001",
"article-title": "Human Genome Sequencing, Finishing the euchromatic sequence of the human genome",
"author": "International",
"doi-asserted-by": "crossref",
"first-page": "931",
"journal-title": "Nature",
"key": "10.1016/j.heliyon.2024.e35331_bib6",
"volume": "431",
"year": "2004"
},
{
"DOI": "10.1093/hmg/9.6.869",
"article-title": "A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes",
"author": "Culetto",
"doi-asserted-by": "crossref",
"first-page": "869",
"journal-title": "Hum. Mol. Genet.",
"key": "10.1016/j.heliyon.2024.e35331_bib7",
"volume": "9",
"year": "2000"
},
{
"DOI": "10.1002/tox.22373",
"article-title": "Reproductive toxicity induced by nickel nanoparticles in Caenorhabditis elegans",
"author": "Kong",
"doi-asserted-by": "crossref",
"first-page": "1530",
"journal-title": "Environ. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib8",
"volume": "32",
"year": "2017"
},
{
"DOI": "10.1093/toxsci/kfn121",
"article-title": "Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology",
"author": "Leung",
"doi-asserted-by": "crossref",
"first-page": "5",
"journal-title": "Toxicol. Sci.",
"key": "10.1016/j.heliyon.2024.e35331_bib9",
"volume": "106",
"year": "2008"
},
{
"DOI": "10.1002/jat.3357",
"article-title": "The C. elegans model in toxicity testing",
"author": "Hunt",
"doi-asserted-by": "crossref",
"first-page": "50",
"journal-title": "J. Appl. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib10",
"volume": "37",
"year": "2017"
},
{
"article-title": "Caenorhabditis elegans, a biological model for research in toxicology",
"author": "Tejeda-Benitez",
"first-page": "1",
"journal-title": "Rev. Environ. Contam. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib11",
"volume": "237",
"year": "2016"
},
{
"DOI": "10.1152/physiolgenomics.00063.2018",
"article-title": "Comparative genomic analysis of the human and nematode Caenorhabditis elegans uncovers potential reproductive genes and disease associations in humans",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1002",
"journal-title": "Physiol. Genom.",
"key": "10.1016/j.heliyon.2024.e35331_bib12",
"volume": "50",
"year": "2018"
},
{
"DOI": "10.1038/nature08283",
"article-title": "An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA",
"author": "Maida",
"doi-asserted-by": "crossref",
"first-page": "230",
"journal-title": "Nature",
"key": "10.1016/j.heliyon.2024.e35331_bib31",
"volume": "461",
"year": "2009"
},
{
"DOI": "10.1111/cas.14618",
"article-title": "RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19",
"author": "Machitani",
"doi-asserted-by": "crossref",
"first-page": "3976",
"journal-title": "Cancer Sci.",
"key": "10.1016/j.heliyon.2024.e35331_bib32",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.1128/MCB.01021-15",
"article-title": "De novo RNA synthesis by RNA-dependent RNA polymerase activity of telomerase reverse transcriptase",
"author": "Maida",
"doi-asserted-by": "crossref",
"first-page": "1248",
"journal-title": "Mol. Cell Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib40",
"volume": "36",
"year": "2016"
},
{
"DOI": "10.1093/genetics/77.1.71",
"article-title": "The genetics of Caenorhabditis elegans",
"author": "Brenner",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Genetics",
"key": "10.1016/j.heliyon.2024.e35331_bib13",
"volume": "77",
"year": "1974"
},
{
"article-title": "CTD for avigan tablets 200 mg",
"key": "10.1016/j.heliyon.2024.e35331_bib14",
"series-title": "PMDA Report on the Deliberation Results",
"year": "2014"
},
{
"author": "GlaxoSmithKline",
"key": "10.1016/j.heliyon.2024.e35331_bib15"
},
{
"key": "10.1016/j.heliyon.2024.e35331_bib16",
"series-title": "Report on the Deliberation Results (Avigan Tablet 200 Mg) in, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau",
"year": "2014"
},
{
"author": "Genentech",
"key": "10.1016/j.heliyon.2024.e35331_bib17"
},
{
"author": "Maruho Co",
"key": "10.1016/j.heliyon.2024.e35331_bib19",
"series-title": "Amenalief Tablets (200mg)",
"year": "2023"
},
{
"author": "Maruho Co",
"key": "10.1016/j.heliyon.2024.e35331_bib20",
"series-title": "Amenalief Tablets (200mg) CTD",
"year": "2020"
},
{
"DOI": "10.1093/jac/5.4.431",
"article-title": "The activity in vitro against herpes virus of 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine), a new antiviral agent",
"author": "Collins",
"doi-asserted-by": "crossref",
"first-page": "431",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.heliyon.2024.e35331_bib22",
"volume": "5",
"year": "1979"
},
{
"DOI": "10.1016/j.antiviral.2016.04.003",
"article-title": "Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds",
"author": "Smee",
"doi-asserted-by": "crossref",
"first-page": "61",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib23",
"volume": "131",
"year": "2016"
},
{
"DOI": "10.1254/jphs.FP0071679",
"article-title": "Phenoxazine derivatives inactivate human cytomegalovirus, herpes simplex virus-1, and herpes simplex virus-2 in vitro",
"author": "Hayashi",
"doi-asserted-by": "crossref",
"first-page": "369",
"journal-title": "J. Pharmacol. Sci.",
"key": "10.1016/j.heliyon.2024.e35331_bib24",
"volume": "106",
"year": "2008"
},
{
"DOI": "10.1167/iovs.10-5671",
"article-title": "In vitro synergism of trifluorothymidine and ganciclovir against HSV-1",
"author": "Hobden",
"doi-asserted-by": "crossref",
"first-page": "830",
"journal-title": "Invest. Ophthalmol. Vis. Sci.",
"key": "10.1016/j.heliyon.2024.e35331_bib25",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1128/AAC.46.4.977-981.2002",
"article-title": "In vitro and in vivo activities of anti-influenza virus compound T-705",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "977",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.heliyon.2024.e35331_bib26",
"volume": "46",
"year": "2002"
},
{
"DOI": "10.3390/v14020234",
"article-title": "The synthesis and anti-cytomegalovirus activity of piperidine-4-carboxamides",
"author": "Guo",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.heliyon.2024.e35331_bib27",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkq198",
"article-title": "ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2",
"author": "Chono",
"doi-asserted-by": "crossref",
"first-page": "1733",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.heliyon.2024.e35331_bib28",
"volume": "65",
"year": "2010"
},
{
"DOI": "10.1073/pnas.83.21.8333",
"article-title": "Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase",
"author": "Furman",
"doi-asserted-by": "crossref",
"first-page": "8333",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.heliyon.2024.e35331_bib29",
"volume": "83",
"year": "1986"
},
{
"DOI": "10.1016/0012-1606(76)90119-6",
"article-title": "The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction",
"author": "Byerly",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Dev. Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib30",
"volume": "51",
"year": "1976"
},
{
"key": "10.1016/j.heliyon.2024.e35331_bib90",
"series-title": "WormAtlas",
"year": "2002"
},
{
"DOI": "10.1016/j.ydbio.2021.01.006",
"article-title": "Human gastrulation: the embryo and its models",
"author": "Ghimire",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "Dev. Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib91",
"volume": "474",
"year": "2021"
},
{
"author": "Nance",
"first-page": "1",
"key": "10.1016/j.heliyon.2024.e35331_bib92",
"series-title": "Gastrulation in C. elegans",
"year": "2005"
},
{
"article-title": "The maternal-to-zygotic transition revisited",
"author": "Vastenhouw",
"first-page": "146",
"journal-title": "Development",
"key": "10.1016/j.heliyon.2024.e35331_bib93",
"year": "2019"
},
{
"DOI": "10.1016/0014-4827(61)90192-6",
"article-title": "The serial cultivation of human diploid cell strains",
"author": "Hayflick",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Exp. Cell Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib33",
"volume": "25",
"year": "1961"
},
{
"DOI": "10.1016/0022-5193(73)90198-7",
"article-title": "A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon",
"author": "Olovnikov",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "J. Theor. Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib34",
"volume": "41",
"year": "1973"
},
{
"DOI": "10.1093/molehr/gat055",
"article-title": "Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species",
"author": "Ozturk",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Mol. Hum. Reprod.",
"key": "10.1016/j.heliyon.2024.e35331_bib35",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1093/molehr/gaq048",
"article-title": "Telomere lengths in human oocytes, cleavage stage embryos and blastocysts",
"author": "Turner",
"doi-asserted-by": "crossref",
"first-page": "685",
"journal-title": "Mol. Hum. Reprod.",
"key": "10.1016/j.heliyon.2024.e35331_bib36",
"volume": "16",
"year": "2010"
},
{
"article-title": "The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: a systematic review",
"author": "Salas-Huetos",
"first-page": "8",
"journal-title": "Cells",
"key": "10.1016/j.heliyon.2024.e35331_bib37",
"year": "2019"
},
{
"DOI": "10.1038/35053110",
"article-title": "Role for a bidentate ribonuclease in the initiation step of RNA interference",
"author": "Bernstein",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nature",
"key": "10.1016/j.heliyon.2024.e35331_bib38",
"volume": "409",
"year": "2001"
},
{
"DOI": "10.1016/S0092-8674(04)00045-5",
"article-title": "MicroRNAs: genomics, biogenesis, mechanism, and function",
"author": "Bartel",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Cell",
"key": "10.1016/j.heliyon.2024.e35331_bib39",
"volume": "116",
"year": "2004"
},
{
"DOI": "10.3390/v13081547",
"article-title": "Amenamevir, a helicase-primase inhibitor, for the optimal treatment of herpes zoster",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.heliyon.2024.e35331_bib41",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104829",
"article-title": "Viral ribonucleotide reductase attenuates the anti-herpes activity of acyclovir in contrast to amenamevir",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib42",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1358/dot.2017.53.11.2724803",
"article-title": "Helicase-primase inhibitor amenamevir for herpesvirus infection: towards practical application for treating herpes zoster",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"first-page": "573",
"journal-title": "Drugs Today",
"key": "10.1016/j.heliyon.2024.e35331_bib43",
"volume": "53",
"year": "2017"
},
{
"DOI": "10.1006/faat.1996.0117",
"article-title": "Nonclinical toxicology studies with zidovudine: reproductive toxicity studies in rats and rabbits",
"author": "Greene",
"doi-asserted-by": "crossref",
"first-page": "140",
"journal-title": "Fund. Appl. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib45",
"volume": "32",
"year": "1996"
},
{
"DOI": "10.1016/j.biopha.2020.110114",
"article-title": "Recent advances in the molecular mechanism of thalidomide teratogenicity",
"author": "Gao",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.heliyon.2024.e35331_bib46",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1073/pnas.91.9.4082",
"article-title": "Thalidomide is an inhibitor of angiogenesis",
"author": "D'Amato",
"doi-asserted-by": "crossref",
"first-page": "4082",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.heliyon.2024.e35331_bib47",
"volume": "91",
"year": "1994"
},
{
"DOI": "10.1126/science.1177319",
"article-title": "Identification of a primary target of thalidomide teratogenicity",
"author": "Ito",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Science",
"key": "10.1016/j.heliyon.2024.e35331_bib48",
"volume": "327",
"year": "2010"
},
{
"DOI": "10.1038/s41589-019-0366-7",
"article-title": "p63 is a cereblon substrate involved in thalidomide teratogenicity",
"author": "Asatsuma-Okumura",
"doi-asserted-by": "crossref",
"first-page": "1077",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib49",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.15252/embj.2020105375",
"article-title": "Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF",
"author": "Yamanaka",
"doi-asserted-by": "crossref",
"journal-title": "EMBO J.",
"key": "10.1016/j.heliyon.2024.e35331_bib50",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1002/bdrb.10054",
"article-title": "Effects of thalidomide on reproductive function and early embryonic development in male and female New Zealand white rabbits",
"author": "Teo",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Birth Defects Res B Dev Reprod Toxicol",
"key": "10.1016/j.heliyon.2024.e35331_bib51",
"volume": "71",
"year": "2004"
},
{
"DOI": "10.1001/jama.1963.63700020034021b",
"article-title": "Congenital malformation in strain A mice. Its experimental production by thalidomide",
"author": "Dipaolo",
"doi-asserted-by": "crossref",
"first-page": "139",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e35331_bib52",
"volume": "183",
"year": "1963"
},
{
"key": "10.1016/j.heliyon.2024.e35331_bib53",
"series-title": "Fujimoto Pharmaceutical Corp, Thaled® Capsule 25/50/100",
"year": "2021"
},
{
"DOI": "10.1124/mol.64.2.415",
"article-title": "Thalidomide down-regulates transcript levels of GC-rich promoter genes in multiple myeloma",
"author": "Drucker",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "Mol. Pharmacol.",
"key": "10.1016/j.heliyon.2024.e35331_bib54",
"volume": "64",
"year": "2003"
},
{
"DOI": "10.1007/BF00316250",
"article-title": "Gross-structural defects in rats after acyclovir application on day 10 of gestation",
"author": "Chahoud",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Arch. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib55",
"volume": "62",
"year": "1988"
},
{
"DOI": "10.1016/0890-6238(93)90079-M",
"article-title": "Significance of embryo culture methods for studying the prenatal toxicity of virustatic agents",
"author": "Stahlmann",
"doi-asserted-by": "crossref",
"first-page": "129",
"issue": "Suppl 1",
"journal-title": "Reprod. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib56",
"volume": "7",
"year": "1993"
},
{
"DOI": "10.1007/BF00293693",
"article-title": "Prenatal toxicity of acyclovir in rats",
"author": "Stahlmann",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "Arch. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib57",
"volume": "61",
"year": "1988"
},
{
"article-title": "Monitoring pregnancy outcomes following prenatal drug exposure through prospective pregnancy registries and passive surveillance: a pharmaceutical company commitment",
"author": "Eldridge",
"first-page": "190",
"journal-title": "Prim. Care Update OB/GYNS",
"key": "10.1016/j.heliyon.2024.e35331_bib58",
"volume": "5",
"year": "1998"
},
{
"DOI": "10.1001/jama.2010.1206",
"article-title": "Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects",
"author": "Pasternak",
"doi-asserted-by": "crossref",
"first-page": "859",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2024.e35331_bib59",
"volume": "304",
"year": "2010"
},
{
"DOI": "10.1007/BF01968962",
"article-title": "In vitro and in vivo studies on the prenatal toxicity of five virustatic nucleoside analogues in comparison to aciclovir",
"author": "Klug",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Arch. Toxicol.",
"key": "10.1016/j.heliyon.2024.e35331_bib60",
"volume": "65",
"year": "1991"
},
{
"DOI": "10.1097/00007890-199903150-00021",
"article-title": "Absence of teratogenicity of oral ganciclovir used during early pregnancy in a liver transplant recipient",
"author": "Pescovitz",
"doi-asserted-by": "crossref",
"first-page": "758",
"journal-title": "Transplantation",
"key": "10.1016/j.heliyon.2024.e35331_bib61",
"volume": "67",
"year": "1999"
},
{
"DOI": "10.1159/000221861",
"article-title": "In vitro and in vivo effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) on types 1 and 3 parainfulenza virus infections",
"author": "Sidwell",
"doi-asserted-by": "crossref",
"first-page": "205",
"journal-title": "Chemotherapy",
"key": "10.1016/j.heliyon.2024.e35331_bib62",
"volume": "21",
"year": "1975"
},
{
"DOI": "10.1097/00006454-200205000-00037",
"article-title": "Safety issues related to the administration of ribavirin",
"author": "Krilov",
"doi-asserted-by": "crossref",
"first-page": "479",
"journal-title": "Pediatr. Infect. Dis. J.",
"key": "10.1016/j.heliyon.2024.e35331_bib63",
"volume": "21",
"year": "2002"
},
{
"DOI": "10.1002/hep.23504",
"article-title": "Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials",
"author": "Awad",
"doi-asserted-by": "crossref",
"first-page": "1176",
"journal-title": "Hepatology",
"key": "10.1016/j.heliyon.2024.e35331_bib64",
"volume": "51",
"year": "2010"
},
{
"DOI": "10.1126/science.401547",
"article-title": "Congenital anomalies induced in hamster embryos with ribavirin",
"author": "Kilham",
"doi-asserted-by": "crossref",
"first-page": "413",
"journal-title": "Science",
"key": "10.1016/j.heliyon.2024.e35331_bib65",
"volume": "195",
"year": "1977"
},
{
"DOI": "10.1016/j.jhep.2009.12.039",
"article-title": "Seminal fluid ribavirin level and functional semen parameters in patients with chronic hepatitis C on antiviral combination therapy",
"author": "Hofer",
"doi-asserted-by": "crossref",
"first-page": "812",
"journal-title": "J. Hepatol.",
"key": "10.1016/j.heliyon.2024.e35331_bib66",
"volume": "52",
"year": "2010"
},
{
"DOI": "10.1016/S1383-5718(01)00308-4",
"article-title": "Ribavirin-induced sperm shape abnormalities in Wistar rat",
"author": "Narayana",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Mutat. Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib67",
"volume": "513",
"year": "2002"
},
{
"DOI": "10.1016/j.fertnstert.2008.07.1755",
"article-title": "Ribavirin and pegylated interferon treatment for hepatitis C was associated not only with semen alterations but also with sperm deoxyribonucleic acid fragmentation in humans",
"author": "Pecou",
"doi-asserted-by": "crossref",
"first-page": "933 e917",
"journal-title": "Fertil. Steril.",
"key": "10.1016/j.heliyon.2024.e35331_bib68",
"volume": "91",
"year": "2009"
},
{
"DOI": "10.1097/00006454-199009001-00008",
"article-title": "Effects of exposure to high concentrations of ribavirin in developing embryos",
"author": "Kochhar",
"doi-asserted-by": "crossref",
"first-page": "S88",
"journal-title": "Pediatr. Infect. Dis. J.",
"key": "10.1016/j.heliyon.2024.e35331_bib69",
"volume": "9",
"year": "1990"
},
{
"DOI": "10.1007/s40264-017-0566-6",
"article-title": "The ribavirin pregnancy registry: an interim analysis of potential teratogenicity at the mid-point of enrollment",
"author": "Sinclair",
"doi-asserted-by": "crossref",
"first-page": "1205",
"journal-title": "Drug Saf.",
"key": "10.1016/j.heliyon.2024.e35331_bib70",
"volume": "40",
"year": "2017"
},
{
"DOI": "10.1371/journal.pntd.0009103",
"article-title": "A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome",
"author": "Suemori",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Neglected Trop. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib71",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103591",
"article-title": "Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome",
"author": "Yuan",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/j.heliyon.2024.e35331_bib72",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/j.pharmthera.2022.108121",
"article-title": "Antiviral therapy for COVID-19: derivation of optimal strategy based on past antiviral and favipiravir experiences",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol. Ther.",
"key": "10.1016/j.heliyon.2024.e35331_bib73",
"volume": "235",
"year": "2022"
},
{
"DOI": "10.1016/j.pharmthera.2020.107512",
"article-title": "Favipiravir, an anti-influenza drug against life-threatening RNA virus infections",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol. Ther.",
"key": "10.1016/j.heliyon.2024.e35331_bib74",
"volume": "209",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib75",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1093/infdis/jiv586",
"article-title": "Efficacy of favipiravir (T-705) in rabies postexposure prophylaxis",
"author": "Yamada",
"doi-asserted-by": "crossref",
"first-page": "1253",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib76",
"volume": "213",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2018.03.003",
"article-title": "Favipiravir as a potential countermeasure against neglected and emerging RNA viruses",
"author": "Delang",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.heliyon.2024.e35331_bib77",
"volume": "153",
"year": "2018"
},
{
"article-title": "Experimental treatment with favipiravir for Ebola virus disease (the jiki trial): a historically controlled, single-arm proof-of-concept trial in Guinea",
"author": "Sissoko",
"journal-title": "PLoS Med.",
"key": "10.1016/j.heliyon.2024.e35331_bib78",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/S1473-3099(15)00228-5",
"article-title": "Post-exposure prophylaxis against Ebola virus disease with experimental antiviral agents: a case-series of health-care workers",
"author": "Jacobs",
"doi-asserted-by": "crossref",
"first-page": "1300",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib79",
"volume": "15",
"year": "2015"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial",
"author": "Ivashchenko",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib80",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1192",
"journal-title": "Engineering",
"key": "10.1016/j.heliyon.2024.e35331_bib81",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiz656",
"article-title": "Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1688",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib82",
"volume": "221",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2022.10.008",
"article-title": "Favipiravir for symptomatic COVID-19: a nationwide observational cohort study",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "J. Infect. Chemother.",
"key": "10.1016/j.heliyon.2024.e35331_bib83",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int. J. Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib84",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"article-title": "Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial",
"author": "Shinkai",
"doi-asserted-by": "crossref",
"journal-title": "Infect. Dis. Ther.",
"key": "10.1016/j.heliyon.2024.e35331_bib85",
"year": "2021"
},
{
"article-title": "Favipiravir versus arbidol for COVID-19: a randomized clinical trial",
"author": "Chen",
"journal-title": "medRxiv",
"key": "10.1016/j.heliyon.2024.e35331_bib86",
"year": "2020"
},
{
"DOI": "10.1016/j.ejogrb.2021.12.001",
"article-title": "Favipiravir exposure and pregnancy outcome of COVID-19 patients",
"author": "Tirmikcioglu",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Eur. J. Obstet. Gynecol. Reprod. Biol.",
"key": "10.1016/j.heliyon.2024.e35331_bib87",
"volume": "268",
"year": "2022"
},
{
"article-title": "The outcomes of favipiravir exposure in pregnancy: a case series",
"author": "Ertem",
"first-page": "1",
"journal-title": "Arch. Gynecol. Obstet.",
"key": "10.1016/j.heliyon.2024.e35331_bib88",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiab247",
"article-title": "beta-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.heliyon.2024.e35331_bib89",
"volume": "224",
"year": "2021"
}
],
"reference-count": 89,
"references-count": 89,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S240584402411362X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}
shiraki