Favipiravir versus Standard of Care in Patients with Severe COVID-19 Infections: A Retrospective Comparative Study
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2021.08.022, Aug 2021
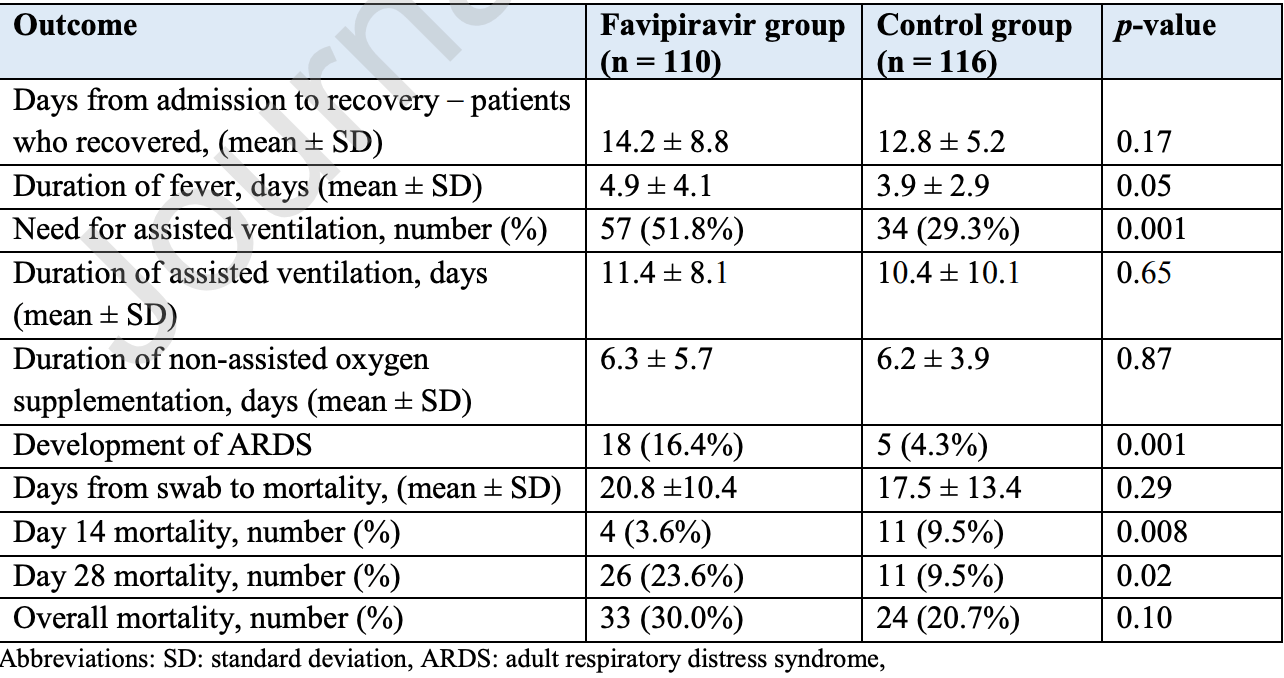
Retrospective 226 COVID-19 pneumonia patients, 110 treated with favipiravir, showing higher mortality (p=0.1) and ICU admission (p=0.02) with treatment in multivariate analysis.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 42.3% higher, RR 1.42, p = 0.10, treatment 33 of 110 (30.0%), control 24 of 116 (20.7%), adjusted per study, odds ratio converted to relative risk, overall mortality, multivariate binary logistic regression.
|
|
risk of death, 149.3% higher, RR 2.49, p = 0.006, treatment 26 of 110 (23.6%), control 11 of 116 (9.5%), day 28.
|
|
risk of death, 61.7% lower, RR 0.38, p = 0.11, treatment 4 of 110 (3.6%), control 11 of 116 (9.5%), NNT 17, day 14.
|
|
risk of ICU admission, 90.0% higher, OR 1.90, p = 0.02, treatment 110, control 116, adjusted per study, multivariate binary logistic regression, RR approximated with OR.
|
|
recovery time, 10.9% higher, relative time 1.11, p = 0.17, treatment 110, control 116.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Almoosa et al., 24 Aug 2021, retrospective, Saudi Arabia, peer-reviewed, 14 authors.
Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study
Journal of Infection and Public Health, doi:10.1016/j.jiph.2021.08.022
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors of the manuscript declared that they have no potential conflicts or competing interests with respect to this research, authorship and/or publication of this article.
Authors' contributions: ZA, MS and GA were involved in writing the proposal, design of the data collection form, and implementation of the study, data analysis, and manuscript preparation. The eight resident doctors were involved in data collection. AA conducted the statistical analysis. ZA, MS GA, and SQ contributed to the manuscript writing. All the team was involved the final revision and edit of the final version of the manuscript.
References
Ali Oruc, Oz, Ozturk, Investigation of the disease process and drug combinations in patients with suspected/confirmed COVID-19 using Favipiravir
Bahadur Shrestha, Budhathoki, Khadka, Prajwol, Shah et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virology Journal volume
Beigel, Remdesivir for the treatment of Covid-19-preliminary report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Cai, Minghui, Dongjingliu, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Chen, Chao, Lai, Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients, J Infect
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial, doi:10.1101/2020.03.17.20037432
Doi, Hibino, Hase, Yamamoto, A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrobial agents and chemotherapy
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Guner, Hasanoglu, Kayaaslan, Aypak, Akinci et al., Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, favipiravir, and hydroxychloroquine plus favipiravir, Journal of Infection and Public Health
Hany, Dabbous, Abd-Elsalam, Manal, El-Sayed et al., Efficacy of favipiravir in COVID-19 treatment: a multicenter randomized study, Archives of Virology volume
Hany, Dabbous, Manal, El-Sayed, Assal et al., Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial, Scientific Reports volume
Hui, Azhar, Madani, Ntoumi, Kock et al., The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health -The latest 2019 J o u r n a l P r e -p r o o f novel coronavirus outbreak in Wuhan, China, Int J Infect Dis, doi:10.1016/j.ijid.2020.01.009.PMID31953166
Irie, Nakagawa, Fujita, Tamura, Eto et al., Pharmacokinetics of Favipiravir in Critically Ill Patients with COVID-19, Clin Transl Sci, doi:10.1111/cts.12827
J O U R N A L P R E, -p r o o f
Khamis, Naabi, Al Lawati, Ambusaidi, Sharji et al., Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.008
Kocayigit, Kezban Ozmen Suner, Tomak, Demir, Observational study of the effects of Favipiravir vs Lopinavir/Ritonavir on clinical outcomes in critically Ill patients with COVID-19, Journal of Clinical Pharmacy and Therapeutics
Kumar, Kulkarni, Sharma, Rao, Reddy, Favipiravir-induced Liver Injury in Patients with Coronavirus Disease 2019, Journal of clinical and translational hepatology, doi:10.14218/JCTH.2021.00011
Manosuthi, Jeungsmarn, Okada, Suwanvattana, Nasopharyngeal SARS-CoV-2 Viral Load Response among COVID-19 Patients Receiving J o u r n a l P r e -p r o o f Favipiravir, Japanese Journal of Infectious Diseases, doi:10.7883/yoken.JJID.2020.827
Sanders, Phd, Monogue, Tomasz, Jodlowski, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19), JAMA, doi:10.1001/jama.2020.6019
Solaymani-Dodaran, Ghanei, Moazen, Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia
The, Group, Dexamethasone in hospitalized patients with Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Wu, Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19, J Intern Med
Xu, Zhao, Teng, Abdalla, Zhu et al., Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV, Viruses, doi:10.3390/v12020244
Zarir, Udwadia, Pawansingh, Hanmantbarkate, Patil et al., MonikaTandon. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, International Journal of Infectious Diseases
Zhang, Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China, Infectious Diseases of Poverty volume
Zhao, Sokhansanj, Malhotra, Zheng, Rosen, Genetic grouping of SARS-CoV-2 coronavirus sequences using informative subtype markers for pandemic spread visualization, PLOS Comp-Biology, doi:10.1371/journal.pcbi.1008269
DOI record:
{
"DOI": "10.1016/j.jiph.2021.08.022",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2021.08.022",
"alternative-id": [
"S1876034121002410"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2021.08.022"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8918-7099",
"affiliation": [],
"authenticated-orcid": false,
"family": "Almoosa",
"given": "Zainab",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saad",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qara",
"given": "Samer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mustafa",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mansour",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshab",
"given": "Duaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alhashem",
"given": "Jehad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ALKhawajah",
"given": "Sajida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkhalifah",
"given": "Saleh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ALmarzooq",
"given": "Mokhtar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ALzain",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anshasi",
"given": "Neda’a",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Gasmelseed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutair",
"given": "Abbas Al",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
24
]
],
"date-time": "2021-08-24T02:59:54Z",
"timestamp": 1629773994000
},
"deposited": {
"date-parts": [
[
2023,
11,
7
]
],
"date-time": "2023-11-07T19:32:44Z",
"timestamp": 1699385564000
},
"indexed": {
"date-parts": [
[
2023,
11,
8
]
],
"date-time": "2023-11-08T00:31:13Z",
"timestamp": 1699403473532
},
"is-referenced-by-count": 10,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
23
]
],
"date-time": "2021-08-23T00:00:00Z",
"timestamp": 1629676800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034121002410?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034121002410?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1247-1253",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jiph.2021.08.022_bib0005",
"unstructured": "Naming the coronavirus disease (COVID-19) and the virus that causes it\". World Health Organization (WHO). Archived from the original on 28 February 2020. Retrieved 28 February 2020."
},
{
"DOI": "10.1016/j.ijid.2020.01.009",
"article-title": "The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China",
"author": "Hui",
"doi-asserted-by": "crossref",
"first-page": "264",
"issue": "February",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2021.08.022_bib0010",
"volume": "91",
"year": "2020"
},
{
"key": "10.1016/j.jiph.2021.08.022_bib0015",
"unstructured": "World Health Organization (WHO) (Press release). 11 March 2020. Retrieved 12 March 2020."
},
{
"DOI": "10.1186/s40249-020-00710-6",
"article-title": "Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang, China",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Infect Dis Poverty",
"key": "10.1016/j.jiph.2021.08.022_bib0020",
"volume": "9",
"year": "2020"
},
{
"article-title": "Genetic grouping of SARS-CoV-2 coronavirus sequences using informative subtype markers for pandemic spread visualization",
"author": "Zhao",
"issue": "September",
"journal-title": "PLOS Comp Biol",
"key": "10.1016/j.jiph.2021.08.022_bib0025",
"year": "2020"
},
{
"DOI": "10.3390/v12020244",
"article-title": "Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "244",
"issue": "February (2)",
"journal-title": "Viruses",
"key": "10.1016/j.jiph.2021.08.022_bib0030",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2021.08.022_bib0035",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19 — preliminary report",
"author": "The RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2021.08.022_bib0040",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19-preliminary report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2021.08.022_bib0045",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "7",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/j.jiph.2021.08.022_bib0050",
"volume": "93",
"year": "2017"
},
{
"article-title": "A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19",
"author": "Doi",
"issue": "November",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jiph.2021.08.022_bib0055",
"year": "2020"
},
{
"article-title": "Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19",
"author": "Wu",
"issue": "July",
"journal-title": "J Intern Med",
"key": "10.1016/j.jiph.2021.08.022_bib0060",
"year": "2020"
},
{
"article-title": "Investigation of the disease process and drug combinations in patients with suspected/confirmed COVID‐19 using Favipiravir",
"author": "Oruc",
"issue": "March",
"journal-title": "J Clin Pract",
"key": "10.1016/j.jiph.2021.08.022_bib0065",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/j.jiph.2020.12.017",
"article-title": "Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, favipiravir, and hydroxychloroquine plus favipiravir",
"author": "Guner",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "March (3)",
"journal-title": "J Infect Public Health",
"key": "10.1016/j.jiph.2021.08.022_bib0070",
"volume": "14",
"year": "2021"
},
{
"article-title": "Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients",
"author": "Chen",
"issue": "December",
"journal-title": "J Infect",
"key": "10.1016/j.jiph.2021.08.022_bib0075",
"year": "2020"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"journal-title": "Virol J",
"key": "10.1016/j.jiph.2021.08.022_bib0080",
"volume": "17",
"year": "2020"
},
{
"article-title": "Favipiravir versus arbidol for COVID-19: a randomized clinical trial",
"author": "Chen",
"first-page": "1199",
"issue": "13",
"journal-title": "medRxiv",
"key": "10.1016/j.jiph.2021.08.022_bib0085",
"volume": "383",
"year": "2021"
},
{
"DOI": "10.1007/s00705-021-04956-9",
"article-title": "Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study",
"author": "Dabbous",
"doi-asserted-by": "crossref",
"first-page": "949",
"journal-title": "Arch Virol",
"key": "10.1016/j.jiph.2021.08.022_bib0090",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1192",
"issue": "October (10)",
"journal-title": "Engineering",
"key": "10.1016/j.jiph.2021.08.022_bib0095",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"issue": "February",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2021.08.022_bib0100",
"volume": "103",
"year": "2021"
},
{
"article-title": "Nasopharyngeal SARS-CoV-2 viral load response among COVID-19 patients receiving favipiravir",
"author": "Manosuthi",
"first-page": "62",
"issue": "103",
"journal-title": "Jpn J Infect Dis",
"key": "10.1016/j.jiph.2021.08.022_bib0105",
"year": "2021"
},
{
"article-title": "Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: a randomised controlled trial",
"author": "Dabbous",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2021.08.022_bib0110",
"volume": "11",
"year": "2021"
},
{
"article-title": "Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia",
"author": "Solaymani-Dodaran",
"issue": "March",
"journal-title": "Int Immunopharmacother",
"key": "10.1016/j.jiph.2021.08.022_bib0115",
"year": "2021"
},
{
"DOI": "10.1111/jcpt.13305",
"article-title": "Observational study of the effects of Favipiravir vs Lopinavir/Ritonavir on clinical outcomes in critically Ill patients with COVID-19",
"author": "Kocayigit",
"doi-asserted-by": "crossref",
"first-page": "454",
"issue": "2",
"journal-title": "J Clin Pharm Ther",
"key": "10.1016/j.jiph.2021.08.022_bib0120",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.008",
"article-title": "Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia",
"author": "Khamis",
"doi-asserted-by": "crossref",
"first-page": "538",
"issue": "January",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2021.08.022_bib0125",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1111/cts.12827",
"article-title": "Pharmacokinetics of Favipiravir in Critically Ill Patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"first-page": "880",
"issue": "September (5)",
"journal-title": "Clin Transl Sci",
"key": "10.1016/j.jiph.2021.08.022_bib0130",
"volume": "13",
"year": "2020"
},
{
"article-title": "Favipiravir-induced liver injury in patients with coronavirus disease 2019",
"author": "Kumar",
"first-page": "276",
"issue": "2",
"journal-title": "J Clin Transl Hepatol",
"key": "10.1016/j.jiph.2021.08.022_bib0135",
"volume": "9",
"year": "2021"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19). April 13, 2020",
"author": "Sanders",
"first-page": "1824",
"issue": "18",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2021.08.022_bib0140",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(03)00806-5",
"article-title": "Effects of chloroquine on viral infections: an old drug against today’s diseases?",
"author": "Savarino",
"doi-asserted-by": "crossref",
"first-page": "722",
"issue": "11",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jiph.2021.08.022_bib0145",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1002/prp2.293",
"article-title": "Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases",
"author": "Al-Bari",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Pharmacol Res Perspect",
"key": "10.1016/j.jiph.2021.08.022_bib0150",
"volume": "5",
"year": "2017"
},
{
"article-title": "Hydroxychloroqquine or chroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis",
"author": "Mehra",
"issue": "May",
"journal-title": "Lancet",
"key": "10.1016/j.jiph.2021.08.022_bib0155",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034121002410"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "14"
}