Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage
et al., Asian Medical Journal and Alternative Medicine, 23:3, Dec 2023
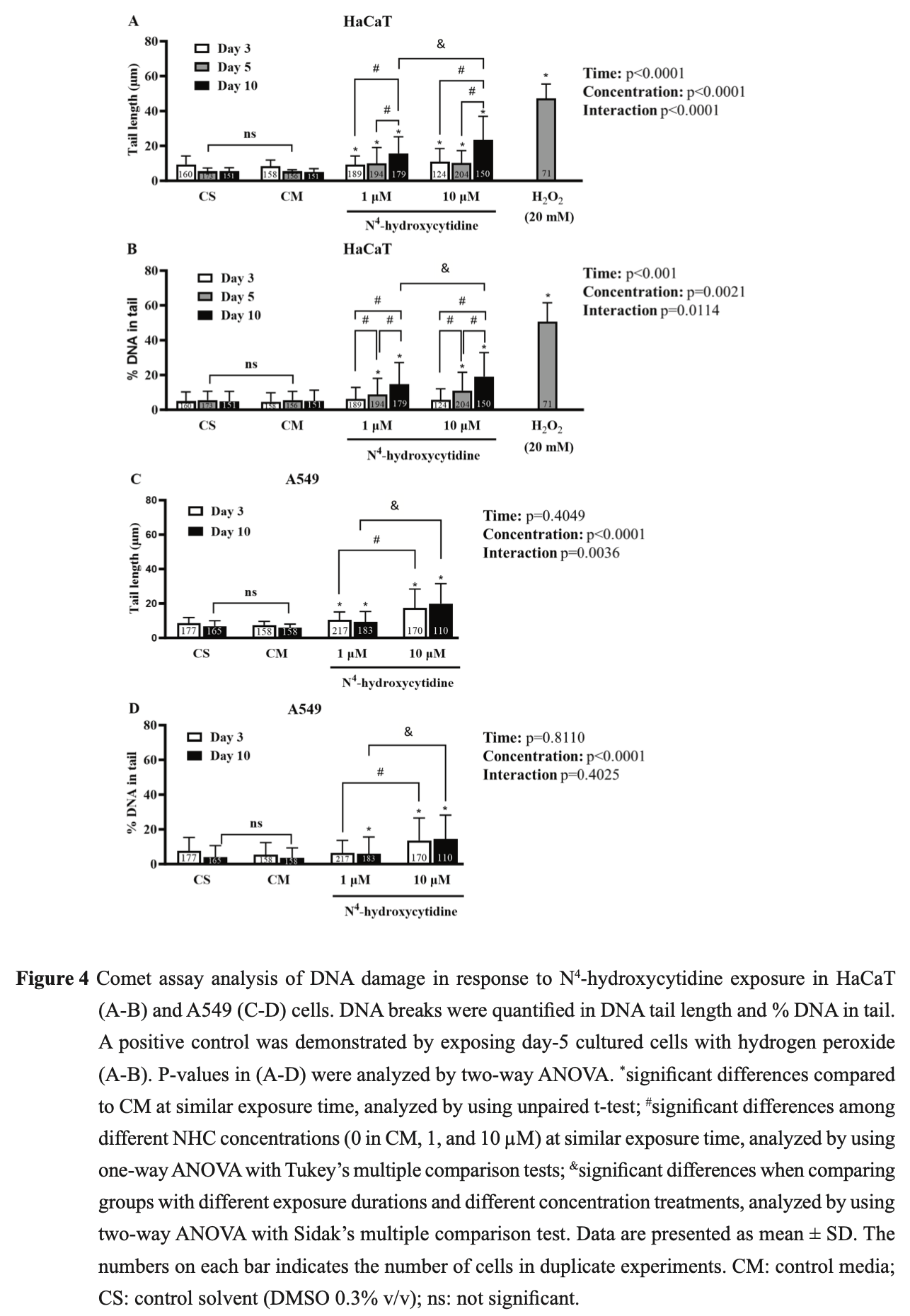
In vitro study showing concentration-dependent cytotoxicity and DNA damage in human skin and lung cell lines with NHC, the active metabolite of molnupiravir, at concentrations spanning the therapeutic range. The genotoxic effects raise concerns about non-specific mutagenesis with clinical use, with increased risk for patients with impaired clearance.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Chamod et al., 28 Dec 2023, peer-reviewed, 9 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Molnupiravir Metabolite--N 4 -hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N 4 -hydroxycytidine Induced Cytotoxicity DNA Damage
N 4 -hydroxycytidine (NHC) is the active metabolite of molnupiravir-a new drug for COVID-19 treatment. NHC exerts antiviral activity by incorporating into SAR-CoV-2 RNA leading to false base-paring and lethal mutations to the virus. However, the risk of non-specific mutagenesis to host cells has been a concern. The goal of this study is to detect cytotoxic activity and DNA damage induced by NHC in rapidgrowing cells including human keratinocyte (HaCaT), and human adenocarcinomic alveolar basal epithelial (A549) cells in vitro by using sulforhodamine B (SRB) colorimetric and comet assays. NHC induced cytotoxicity in a concentration-dependent manner (0.1-30µM) in HaCaT and A549 cells. Half-maximal inhibitory concentration (IC 50 ) values of NHC were lower in HaCaT compared to A549 cells after 3, 5, 10 days of exposure (4.40 ± 0.09 vs 23.21 ± 3.42, 5.82 ± 0.91 vs 16.35 ± 2.04, and 5.41 ± 0.88 vs 13.83 ± 2.05 µM, respectively), suggesting that the cytotoxic effect of NHC is more potent in HaCaT cells than in A549 cells. Significant increase in DNA damage parameters were observed in comet assay for HaCaT and A549 cells after exposure to NHC. NHC-induced DNA damage in HaCaT cells was concentration-dependent (1-10 µM), and time-dependent (3-10 days). NHC-induced DNA damage in A549 cells was concentration-dependent (1-10 µM), but not time-dependent (3-10 days). Within the limitations of this in vitro study, we conclude that NHC could induce cytotoxic and DNA damage in mammalian cells at therapeutic and supratherapeutic concentrations. We propose caution in the use and supervision of molnupiravir, especially in patients with impaired xenobiotic clearance.
Author Contributions Participated in research design: Chamod, Sangsiri, Prajuabjinda, Poomirat, Tangjittham Conducted experiments: Chamod, Poomirat, Prajuabkinda, Tangjittham, Liu, Mongkhonsakunrit, Pakotiprapha, Rimdusit Performed data analysis: Chamod, Sangsiri, Rimdusit Wrote or contributed to the writing of the manuscript: Chamod, Poomirat, Prajuabjinda, Sangsiri
References
Alfarouk, Stock, Taylor, Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp, Cancer Cell Int
Angélica, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine
Annex, Conditions of use, condition for distribution and patients targeted and conditions for safety monitoring adressed to member states for unauthorised product Lagevrio (molnupiravir)
Bajpayee, Kumar, Dhawan, The Comet Assay: Assessment of In Vitro and In Vivo DNA Damage, Methods Mol Biol
Bian, Sabri, Abdulkarim, Interactions between COVID-19 and Lung Cancer: Lessons Learned during the Pandemic, Cancers
Collins, The comet assay for DNA damage and repair: principles, applications, and limitations, Mol Biotechnol
Correia, Fernandes, Cutaneous adverse reactions to the new oral antiviral drugs against SARS-CoV-2, Clin Exp Dermatol
Fischer, Eron, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Fitzgerald, Dickinson, Else, Pharmacokinetics of ß-d-N4-Hydroxycytidine, the Parent Nucleoside of Prodrug Molnupiravir, in Nonplasma Compartments of Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection, Clinical Infectious Diseases
Githaka, Molnupiravir Does Not Induce Mutagenesis in Host Lung Cells during SARS-CoV-2 Treatment, Bioinform Biol Insights
Gordon, Tchesnokov, Schinazi, Götte, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J Biol Chem
Houghton, Fang, Techatanawat, Steventon, Hylands et al., The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity, Methods
Itharat, Houghton, Eno-Amooquaye, Burke, Sampson et al., In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer, J Ethnopharmacol
Kabinger, Stiller, Schmitzová, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nature Structural & Molecular Biology
Keeratichamroen, Lirdprapamongkol, Svasti, Mechanism of ECM-induced dormancy and chemoresistance in A549 human lung carcinoma cells, Oncol Rep
Kobayashi, Mori, Ahmed, Oxidative DNA Damage by N4-hydroxycytidine, a Metabolite of the SARS-CoV-2 Antiviral Molnupiravir, The Journal of Infectious Diseases
Law, Ho, Law, Cheung, Gastrointestinal and hepatic side effects of potential treatment for COVID-19 and vaccination in patients with chronic liver diseases, World J Hepatol
Li, Lei, Yao, Autophagy and multidrug resistance in cancer, Chinese Journal of Cancer
Liao, Mcnutt, Zhu, The comet assay: A sensitive method for detecting DNA damage in individual cells, Methods
Linn, DNA damage by iron and hydrogen peroxide in vitro and in vivo, Drug Metab Rev
Lu, Liu, Yang, Evaluating In Vitro DNA Damage Using Comet Assay, J Vis Exp
Miranda, Mckinzie, Dobrovolsky, Revollo, Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial and mammalian cells by HiFi sequencing, Environmental and Molecular Mutagenesis
Olive, Banáth, The comet assay: a method to measure DNA damage in individual cells, Nature Protocols
Painter, Holman, Bush, Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.02428-02420
Petersen, Gniadecki, Vicanova, Thorn, Wulf, Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes, Journal of Photochemistry and Photobiology B: Biology
Santi Laurini, Montanaro, Motola, Safety Profile of Molnupiravir in the Treatment of COVID-19: A Descriptive Study Based on FAERS Data, J Clin Med
Singh, Mccoy, Tice, Schneider, A simple technique for quantitation of low levels of DNA damage in individual cells, Exp Cell Res
Singh, Singh, Singh, Misra, An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19, Diabetes & Metabolic Syndrome: Clinical Research & Reviews
Skehan, Storeng, Scudiero, New colorimetric cytotoxicity assay for anticancer-drug screening, J Natl Cancer Inst
Speit, Hartmann, The comet assay: a sensitive genotoxicity test for the detection of DNA damage, Methods Mol Biol
Troth, Butterton, Deanda, Letter to the Editor in Response to Zhou et al, J Infect Dis
Vichai, Kirtikara, Sulforhodamine B colorimetric assay for cytotoxicity screening, Nature Protocols
Wallace, Bjork, Molnupiravir; molecular and functional descriptors of mitochondrial safety, Toxicol Appl Pharmacol
Whitley, Molnupiravir -A Step toward Orally Bioavailable Therapies for Covid-19, New England Journal of Medicine
Zhao, He, Huang, A novel model of molnupiravir against SARS-CoV-2 replication: accumulated RNA mutations to induce error catastrophe, Signal Transduction and Targeted Therapy
Zhou, Hill, Sarkar, β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, J Infect Dis