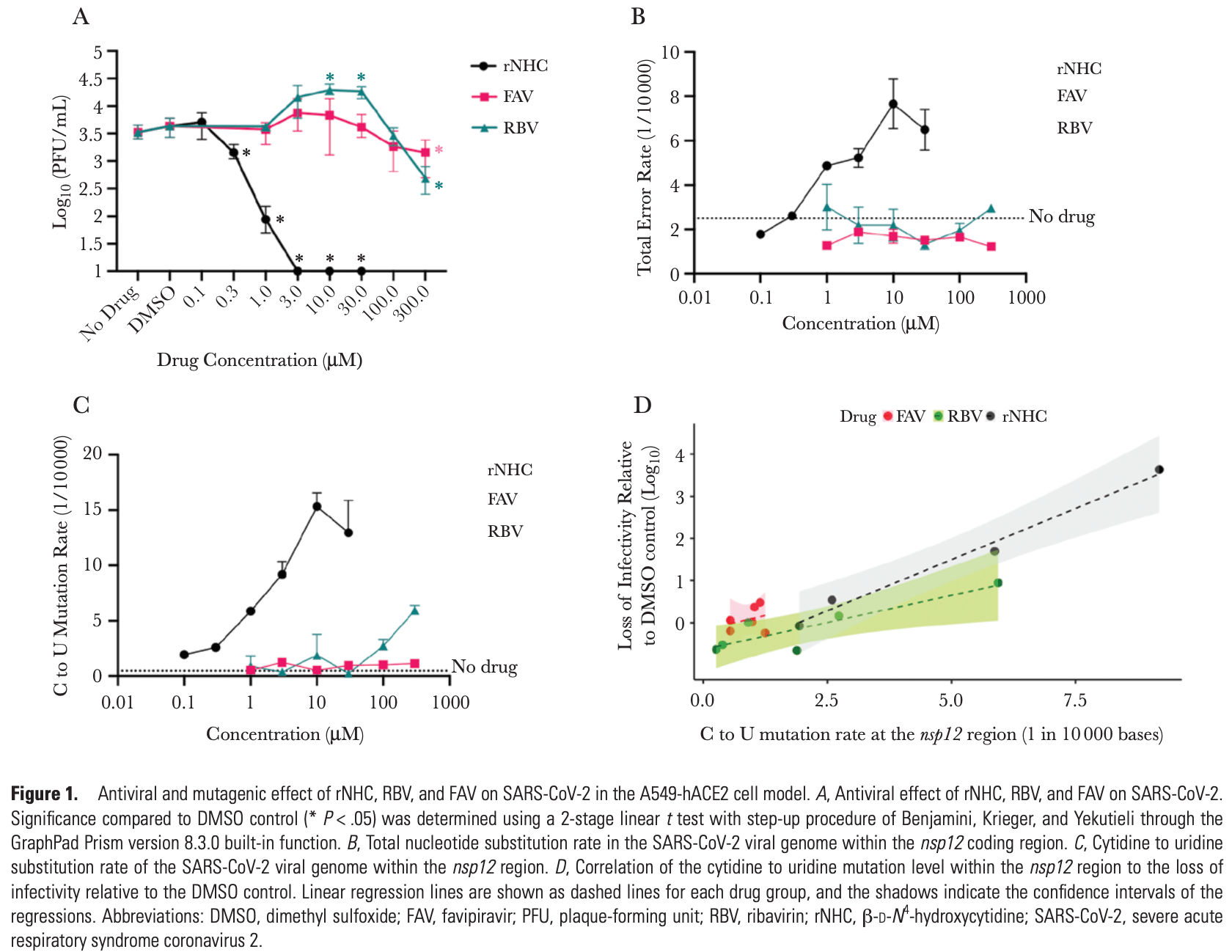
β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247, May 2021
In vitro study showing that NHC (initial metabolite of molnupiravir) has high antiviral activity against SARS-CoV-2, but also shows host mutational activity in an animal cell culture assay. Authors note the concern that mutations in host DNA could contribute to the development of cancer, or cause birth defects either in a developing fetus or through incorporation into sperm precursor cells. Response from Merck:1.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity2-16. Multiple analyses have identified variants potentially created by molnupiravir17-21. Studies show significantly increased risk of acute kidney injury22, cardiovascular toxocity23, and neurological symptoms22. Treatment may increase viral rebound24,25.
2.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
3.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
4.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
5.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
6.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
7.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
8.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
9.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
10.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
11.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
12.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
13.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
14.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
15.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
16.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
17.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
18.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
19.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
20.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
22.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
23.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Zhou et al., 7 May 2021, peer-reviewed, 10 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Abstract: The Journal of Infectious Diseases
Brief Report
β-d-N4-hydroxycytidine Inhibits SARSCoV-2 Through Lethal Mutagenesis But
Is Also Mutagenic To Mammalian Cells
Shuntai Zhou,1, Collin S. Hill,1 Sanjay Sarkar,2 Longping V. Tse,3
Blaide M. D. Woodburn,1,4 Raymond F. Schinazi,5 Timothy P. Sheahan,3
Ralph S. Baric,3,6 Mark T. Heise,2,6 and Ronald Swanstrom1,7
Mutagenic ribonucleosides can act as broad-based antiviral
agents. They are metabolized to the active ribonucleoside triphosphate form and concentrate in genomes of RNA viruses
during viral replication. β-d-N4-hydroxycytidine (NHC, initial metabolite of molnupiravir) is >100-fold more active than
ribavirin or favipiravir against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with antiviral activity
correlated to the level of mutagenesis in virion RNA. However,
NHC also displays host mutational activity in an animal cell
culture assay, consistent with RNA and DNA precursors sharing
a common intermediate of a ribonucleoside diphosphate. These
results indicate highly active mutagenic ribonucleosides may
hold risk for the host.
Keywords.
molnupiravir;
mutagenicity;
NHC;
SARS-CoV-2.
Emerging RNA viruses arising from highly heterogeneous
pools of precursor strains are responsible for most recent epidemic and pandemic disease outbreaks in the late 20th and early
21st century. Broad direct-acting antiviral agents represent the
most specific way of treating a viral infection, although these
are often specific to a group of closely related viruses. Besides
remdesivir, which blocks the replication of several coronaviruses, limited therapeutic options are available for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
infections [1]. β-d-N4-hydroxycytidine (NHC, or to designate
Received 18 February 2021; editorial decision 1 May 2021; accepted 4 May 2021; published
online May 7, 2021.
Correspondence: Ronald Swanstrom, PhD, Room 22-006, Lineberger Comprehensive Cancer
Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599 (risunc@med.unc.
edu).
The Journal of Infectious Diseases® 2021;224:415–9
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society
of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
DOI: 10.1093/infdis/jiab247
METHODS
We measured SARS-CoV-2 antiviral activity in the presence
of a panel of compounds (rNHC, RBV, and FAV) using the
A549-hACE2 cell model [9, 10]. Sequence analysis to detect the
mutation load was done using the previously published multiplex Primer ID approach to sequence several regions of the
BRIEF REPORT • jid 2021:224 (1 August) • 415
1
Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel
Hill, North Carolina, USA, 2Department of Genetics, University of North Carolina at Chapel
Hill, Chapel Hill, North Carolina, USA, 3Department of Epidemiology, University of North
Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, 4Department of Pharmacology,
University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, 5Laboratory of
Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine
and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA, 6Department of Microbiology
and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North..
DOI record:
{
"DOI": "10.1093/infdis/jiab247",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiab247",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Mutagenic ribonucleosides can act as broad-based antiviral agents. They are metabolized to the active ribonucleoside triphosphate form and concentrate in genomes of RNA viruses during viral replication. β-d-N4-hydroxycytidine (NHC, initial metabolite of molnupiravir) is &gt;100-fold more active than ribavirin or favipiravir against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with antiviral activity correlated to the level of mutagenesis in virion RNA. However, NHC also displays host mutational activity in an animal cell culture assay, consistent with RNA and DNA precursors sharing a common intermediate of a ribonucleoside diphosphate. These results indicate highly active mutagenic ribonucleosides may hold risk for the host.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8353-386X",
"affiliation": [
{
"name": "Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"authenticated-orcid": false,
"family": "Zhou",
"given": "Shuntai",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Hill",
"given": "Collin S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Sarkar",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Tse",
"given": "Longping V",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
},
{
"name": "Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Woodburn",
"given": "Blaide M D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Biochemical Pharmacology, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, Georgia, USA"
}
],
"family": "Schinazi",
"given": "Raymond F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Sheahan",
"given": "Timothy P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
},
{
"name": "Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Baric",
"given": "Ralph S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
},
{
"name": "Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Heise",
"given": "Mark T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
},
{
"name": "Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA"
}
],
"family": "Swanstrom",
"given": "Ronald",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
5
]
],
"date-time": "2021-05-05T13:25:20Z",
"timestamp": 1620221120000
},
"deposited": {
"date-parts": [
[
2022,
12,
26
]
],
"date-time": "2022-12-26T11:28:44Z",
"timestamp": 1672054124000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"P30 CA16068",
"P30 AI50410",
"R01 AI140970",
"R01 AI141327",
"P30 AI050409"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"award": [
"U19 AI142759"
],
"name": "Antiviral Drug Discovery and Development Center"
},
{
"DOI": "10.13039/100011072",
"doi-asserted-by": "publisher",
"name": "UNC Center for AIDS Research"
},
{
"DOI": "10.13039/100008615",
"doi-asserted-by": "publisher",
"name": "UNC Lineberger Comprehensive Cancer Center"
},
{
"name": "Emory Center for AIDS"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T09:48:25Z",
"timestamp": 1715680105947
},
"is-referenced-by-count": 219,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
5,
7
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021,
5,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
8,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
7
]
],
"date-time": "2021-05-07T00:00:00Z",
"timestamp": 1620345600000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiab247/38530589/jiab247.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/224/3/415/39541894/jiab247.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/224/3/415/39541894/jiab247.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "415-419",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
5,
7
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
7
]
]
},
"published-other": {
"date-parts": [
[
2021,
8,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
8,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"article-title": "Remdesivir in coronavirus disease 2019 (COVID-19) treatment: a review of evidence",
"author": "Lin",
"journal-title": "Infection",
"key": "2021080216301988800_CIT0001"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"article-title": "An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"first-page": "eabb5883",
"journal-title": "Sci Transl Med",
"key": "2021080216301988800_CIT0002",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01348-19",
"article-title": "Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance",
"author": "Agostini",
"doi-asserted-by": "crossref",
"first-page": "e01348",
"journal-title": "J Virol",
"key": "2021080216301988800_CIT0003",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Nature",
"key": "2021080216301988800_CIT0004",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human Safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "2021080216301988800_CIT0005",
"year": "2021"
},
{
"article-title": "A review on favipiravir: the properties, function, and usefulness to treat COVID-19",
"author": "Hashemian",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "2021080216301988800_CIT0006"
},
{
"DOI": "10.1073/pnas.111085598",
"article-title": "RNA virus error catastrophe: direct molecular test by using ribavirin",
"author": "Crotty",
"doi-asserted-by": "crossref",
"first-page": "6895",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "2021080216301988800_CIT0007",
"volume": "98",
"year": "2001"
},
{
"DOI": "10.1007/978-1-61779-421-6_4",
"article-title": "Mammalian cell HPRT gene mutation assay: test methods",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Methods Mol Biol",
"key": "2021080216301988800_CIT0008",
"volume": "817",
"year": "2012"
},
{
"DOI": "10.1126/science.abe8499",
"article-title": "SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "1464",
"journal-title": "Science",
"key": "2021080216301988800_CIT0009",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"article-title": "SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "429",
"journal-title": "Cell",
"key": "2021080216301988800_CIT0010",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01652-20",
"article-title": "Repurposing nucleoside analogs for human coronaviruses",
"author": "Zandi",
"doi-asserted-by": "crossref",
"first-page": "e01652",
"journal-title": "Antimicrob Agents Chemother",
"key": "2021080216301988800_CIT0011",
"volume": "65",
"year": "2020"
},
{
"article-title": "Biosynthesis of nucleotides.",
"author": "Stryer",
"edition": "4",
"first-page": "739",
"key": "2021080216301988800_CIT0012",
"volume-title": "Biochemistry",
"year": "1995"
},
{
"DOI": "10.1186/s13059-016-0963-7",
"article-title": "A comprehensive survey of the mutagenic impact of common cancer cytotoxics",
"author": "Szikriszt",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Genome Biol",
"key": "2021080216301988800_CIT0013",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1016/j.ijid.2020.03.017",
"article-title": "Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Int J Infect Dis",
"key": "2021080216301988800_CIT0014",
"volume": "94",
"year": "2020"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/224/3/415/6272009"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "β-<scp>d</scp>-<i>N</i>4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells",
"type": "journal-article",
"volume": "224"
}