Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial
et al., The Lancet, doi:10.1016/S0140-6736(22)02597-1, PANORAMIC, ISRCTN30448031, Oct 2022 (preprint)
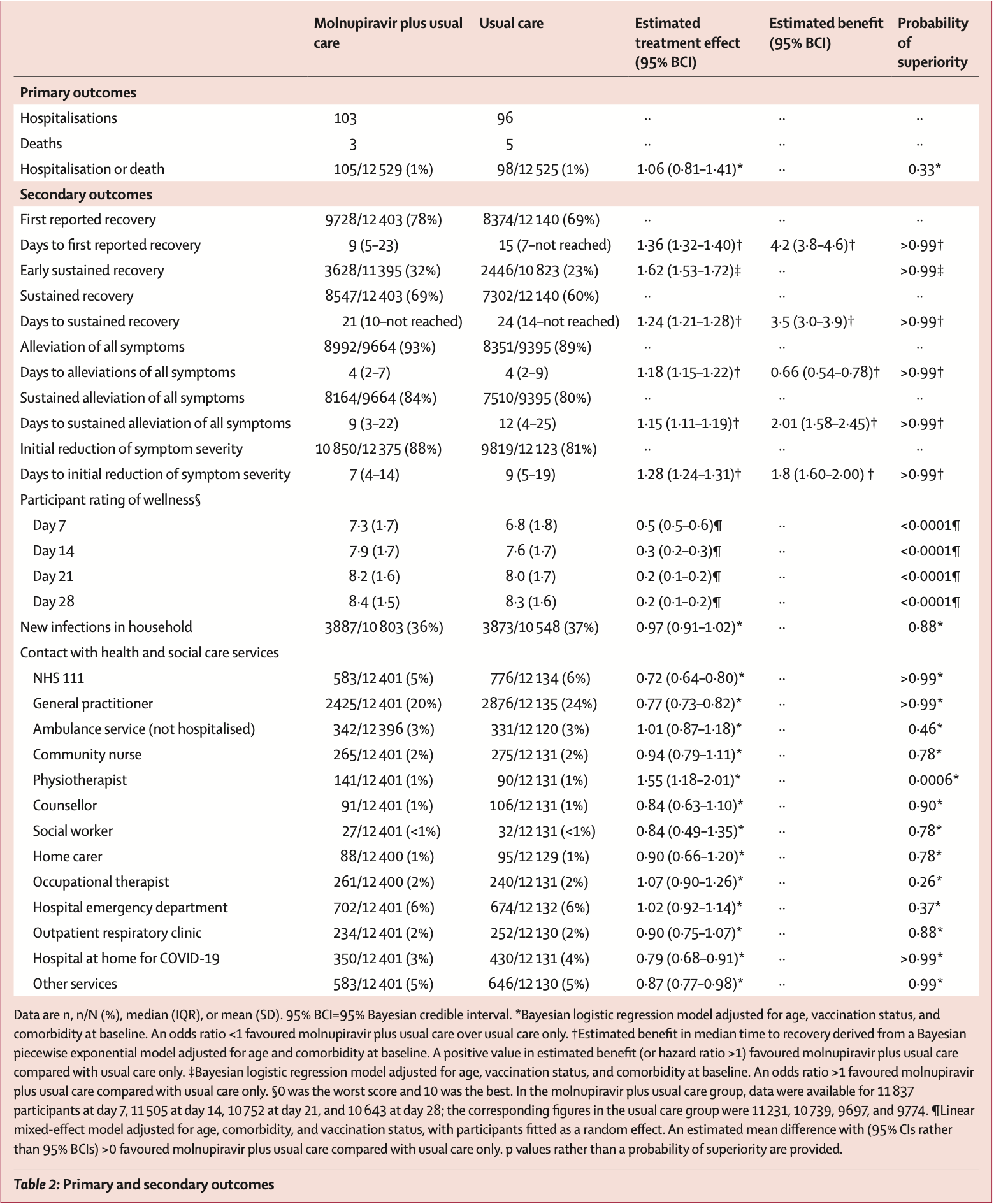
26,411 patient RCT in the UK, showing faster recovery but no significant difference in hospitalization/death or transmission. Improved recovery may be in part due to the open label design with self-reported symptomatic data. Viral load initially declined more quickly, but was higher at 14 days. Longer-term results are from1.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity2-16. Multiple analyses have identified variants potentially created by molnupiravir17-21. Studies show significantly increased risk of acute kidney injury22, cardiovascular toxocity23, and neurological symptoms22. Treatment may increase viral rebound24,25.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments26.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 27.1% higher, RR 1.27, p = 0.50, treatment 20 of 11,778 (0.2%), control 15 of 11,230 (0.1%), day 180.
|
|
risk of death, 40.0% lower, RR 0.60, p = 0.51, treatment 3 of 12,529 (0.0%), control 5 of 12,525 (0.0%), NNT 6260, day 28.
|
|
risk of death/hospitalization, 6.0% higher, RR 1.06, p = 0.69, treatment 105 of 12,529 (0.8%), control 98 of 12,525 (0.8%), odds ratio converted to relative risk, day 28, primary outcome.
|
|
risk of hospitalization, 7.3% higher, RR 1.07, p = 0.67, treatment 103 of 12,529 (0.8%), control 96 of 12,525 (0.8%), day 28.
|
|
risk of transmission, 1.9% lower, RR 0.98, p = 0.88, treatment 3,887 of 10,803 (36.0%), control 3,873 of 10,548 (36.7%), NNT 136, odds ratio converted to relative risk.
|
|
risk of no sustained recovery, 22.0% lower, RR 0.78, p < 0.001, treatment 3,856 of 12,403 (31.1%), control 4,838 of 12,140 (39.9%), NNT 11.
|
|
recovery time, 19.4% lower, relative time 0.81, p < 0.001, treatment 12,403, control 12,140, inverted to make RR<1 favor treatment, sustained recovery.
|
|
risk of no first recovery, 30.5% lower, RR 0.70, p < 0.001, treatment 2,675 of 12,403 (21.6%), control 3,766 of 12,140 (31.0%), NNT 11.
|
|
recovery time, 26.5% lower, relative time 0.74, p < 0.001, treatment 12,403, control 12,140, inverted to make RR<1 favor treatment, first recovery.
|
|
risk of viral load, 95.0% higher, RR 1.95, p = 0.01, treatment 21, control 11, all samples, day 14.
|
|
risk of viral load, 92.0% lower, RR 0.08, p < 0.001, treatment 33, control 26, all samples, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Harris et al., Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00431-6.
2.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
3.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
4.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
5.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
6.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
7.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
8.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
9.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
10.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
11.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
12.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
13.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
14.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
15.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
16.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
17.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
18.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
19.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
20.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
22.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
23.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
24.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Butler et al., 6 Oct 2022, Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 56.6, 135 authors, study period 8 December, 2021 - 27 April, 2022, average treatment delay 3.0 days, trial ISRCTN30448031 (PANORAMIC).
Contact: christopher.butler@phc.ox.
Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial
The Lancet, doi:10.1016/s0140-6736(22)02597-1
Background The safety, effectiveness, and cost-effectiveness of molnupiravir, an oral antiviral medication for SARS-CoV-2, has not been established in vaccinated patients in the community at increased risk of morbidity and mortality from COVID-19. We aimed to establish whether the addition of molnupiravir to usual care reduced hospital admissions and deaths associated with COVID-19 in this population. Methods PANORAMIC was a UK-based, national, multicentre, open-label, multigroup, prospective, platform adaptive randomised controlled trial. Eligible participants were aged 50 years or older-or aged 18 years or older with relevant comorbidities-and had been unwell with confirmed COVID-19 for 5 days or fewer in the community. Participants were randomly assigned (1:1) to receive 800 mg molnupiravir twice daily for 5 days plus usual care or usual care only. A secure, web-based system (Spinnaker) was used for randomisation, which was stratified by age (<50 years vs ≥50 years) and vaccination status (yes vs no). COVID-19 outcomes were tracked via a self-completed online daily diary for 28 days after randomisation. The primary outcome was all-cause hospitalisation or death within 28 days of randomisation, which was analysed using Bayesian models in all eligible participants who were randomly assigned. This trial is registered with ISRCTN, number 30448031.
References
Abdelnabi, Foo, Kaptein, The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for COVID-19, BMJ
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Burki, Omicron variant and booster COVID-19 vaccines, Lancet Respir Med
Butler, Yu, Dorward, Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, openlabel, adaptive platform trial, Lancet Respir Med
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults, NEJM Evidence
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Dorward, Yu, Hayward, Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract
Gastine, Pang, Boshier, Systematic review and patientlevel meta-analysis of SARS-CoV-2 viral dynamics to model response to antiviral therapies, Clin Pharmacol Ther
Hayward, Butler, Yu, Platform randomised trial of interventions against COVID-19 in older people (PRINCIPLE): protocol for a randomised, controlled, open-label, adaptive platform, trial of community treatment of COVID-19 syndromic illness in people at higher risk, BMJ Open
Khoo, Fitzgerald, Fletcher, Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase I, openlabel, dose-escalating, randomized controlled study, J Antimicrob Chemother
Khoo, Fitzgerald, Saunders, Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00644-2
Laouénan, Guedj, Mentré, Clinical trial simulation to evaluate power to compare the antiviral effectiveness of two hepatitis C protease inhibitors using nonlinear mixed effect models: a viral kinetic approach, BMC Med Res Methodol
Lawrence, Mirchandani, Hill, Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13 694 patients, doi:10.21203/rs.3.rs-1913200/v1(preprint
Little, Rumsby, Kelly, Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial, JAMA
Little, Stuart, Moore, Amoxicillin for acute lowerrespiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial, Lancet Infect Dis
Lowe, Brown, Chowdhury, Favipiravir, lopinavirritonavir or combination therapy (FLARE): a randomised, double blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19, PLoS Med
Macpherson, Pragmatic clinical trials, Complement Ther Med
Malone, Campbell, Molnupiravir: coding for catastrophe, Nat Struc Mol Biol
Merck, Merck and Ridgeback's investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate COVID-19 in positive interim analysis of phase 3 study
Painter, Holman, Bush, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother
Rosenke, Hansen, Schwarz, Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model, Nat Commun
Siemieniuk, Bartoszko, Ge, Drug treatments for COVID-19: living systematic review and network meta-analysis, BMJ
Singh, Mitra, Arora, Two Indian drugmakers to end trials of generic Merck pill for moderate COVID-19
Thorlund, Sheldrick, Meyerowitz-Katz, Singh, Hill, Making statistical sense of the molnupiravir MOVe-OUT clinical trial, Am J Trop Med Hyg
Urakova, Kuznetsova, Crossman, β-d-N 4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome, J Virol
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Wendler, Kington, Madans, Are racial and ethnic minorities less willing to participate in health research?, PLoS Med
Woodcock, Lavange, Master protocols to study multiple therapies, multiple diseases, or both, N Engl J Med
DOI record:
{
"DOI": "10.1016/s0140-6736(22)02597-1",
"ISSN": [
"0140-6736"
],
"URL": "http://dx.doi.org/10.1016/S0140-6736(22)02597-1",
"alternative-id": [
"S0140673622025971"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S0140-6736(22)02597-1"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S0140-6736(22)02593-4"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Butler",
"given": "Christopher C",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hobbs",
"given": "F D Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gbinigie",
"given": "Oghenekome A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Najib M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayward",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richards",
"given": "Duncan B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dorward",
"given": "Jienchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lowe",
"given": "David M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Standing",
"given": "Joseph F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breuer",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khoo",
"given": "Saye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petrou",
"given": "Stavros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hood",
"given": "Kerenza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen-Van-Tam",
"given": "Jonathan S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahendra G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saville",
"given": "Benjamin R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marion",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogburn",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rutter",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francis",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Nicholas P B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dobson",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madden",
"given": "Tracie-Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holmes",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Png",
"given": "May Ee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lown",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Hecke",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detry",
"given": "Michelle A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Christina T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Nicholas S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mwandigha",
"given": "Lazaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galal",
"given": "Ushma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mort",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jani",
"given": "Bhautesh D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hart",
"given": "Nigel D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Haroon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKenna",
"given": "Micheal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chalk",
"given": "Jem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavallee",
"given": "Layla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadley",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cureton",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benysek",
"given": "Magdalena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andersson",
"given": "Monique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coates",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrett",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bateman",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Jennifer C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raymundo-Wood",
"given": "Ivy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carson-Stevens",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Ly-Mee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Little",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agyeman",
"given": "Akosua A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Tanveer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allcock",
"given": "Damien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beltran-Martinez",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benedict",
"given": "Oluseye E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bird",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brennan",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Julianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burns",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Zelda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danson",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Kare-Silver",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhasmana",
"given": "Devesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dickson",
"given": "Jon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engamba",
"given": "Serge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fisher",
"given": "Stacey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frost",
"given": "Eve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaunt",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghosh",
"given": "Sarit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilkar",
"given": "Ishtiaq",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Granier",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Howell",
"given": "Aleksandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussain",
"given": "Iqbal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutchinson",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imlach",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Irving",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobsen",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kennard",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Umar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knox",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krasucki",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Law",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Rem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lester",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lunn",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mackintosh",
"given": "Claire I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathukia",
"given": "Mehul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morton",
"given": "Seb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nally",
"given": "Rhiannon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ndukauba",
"given": "Chinonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogundapo",
"given": "Olufunto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okeke",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Kavil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Penfold",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poonian",
"given": "Satveer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Popoola",
"given": "Olajide",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pora",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Vibhore",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Rishabh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Razzaq",
"given": "Omair",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Scot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Royal",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Safa",
"given": "Afsana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sehdev",
"given": "Satash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sevenoaks",
"given": "Tamsin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheikh",
"given": "Aadil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Short",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sidhu",
"given": "Baljinder S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Ivor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soni",
"given": "Yusuf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thalasselis",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "Pete",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wingfield",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woodall",
"given": "Maximillian N J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wooding",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woods",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yong",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yongblah",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zafar",
"given": "Azhar",
"sequence": "additional"
}
],
"container-title": "The Lancet",
"container-title-short": "The Lancet",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
22
]
],
"date-time": "2022-12-22T23:41:15Z",
"timestamp": 1671752475000
},
"deposited": {
"date-parts": [
[
2022,
12,
22
]
],
"date-time": "2022-12-22T23:41:29Z",
"timestamp": 1671752489000
},
"indexed": {
"date-parts": [
[
2022,
12,
23
]
],
"date-time": "2022-12-23T15:57:04Z",
"timestamp": 1671811024762
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 19,
"start": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T00:00:00Z",
"timestamp": 1671494400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0140673622025971?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0140673622025971?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1126/scitranslmed.aax5866",
"article-title": "Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia",
"author": "Toots",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S0140-6736(22)02597-1_bib1",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "Fischer",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S0140-6736(22)02597-1_bib2",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41594-021-00657-8",
"article-title": "Molnupiravir: coding for catastrophe",
"author": "Malone",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Nat Struc Mol Biol",
"key": "10.1016/S0140-6736(22)02597-1_bib3",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"article-title": "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Nat Microbiol",
"key": "10.1016/S0140-6736(22)02597-1_bib4",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Nature",
"key": "10.1016/S0140-6736(22)02597-1_bib5",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-22580-8",
"article-title": "Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model",
"author": "Rosenke",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S0140-6736(22)02597-1_bib6",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkab318",
"article-title": "Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase I, open-label, dose-escalating, randomized controlled study",
"author": "Khoo",
"doi-asserted-by": "crossref",
"first-page": "3286",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S0140-6736(22)02597-1_bib7",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"first-page": "e02428",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S0140-6736(22)02597-1_bib8",
"volume": "65",
"year": "2021"
},
{
"article-title": "Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults",
"author": "Caraco",
"journal-title": "NEJM Evidence",
"key": "10.1016/S0140-6736(22)02597-1_bib9",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/S0140-6736(22)02597-1_bib10",
"volume": "386",
"year": "2022"
},
{
"author": "Singh",
"key": "10.1016/S0140-6736(22)02597-1_bib11"
},
{
"DOI": "10.1016/S1473-3099(22)00644-2",
"article-title": "Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial",
"author": "Khoo",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S0140-6736(22)02597-1_bib12",
"year": "2022"
},
{
"DOI": "10.1056/NEJMra1510062",
"article-title": "Master protocols to study multiple therapies, multiple diseases, or both",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "N Engl J Med",
"key": "10.1016/S0140-6736(22)02597-1_bib13",
"volume": "377",
"year": "2017"
},
{
"key": "10.1016/S0140-6736(22)02597-1_bib14",
"series-title": "COVID-19 rapid guideline: managing COVID-19",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2223",
"article-title": "Systematic review and patient-level meta-analysis of SARS-CoV-2 viral dynamics to model response to antiviral therapies",
"author": "Gastine",
"doi-asserted-by": "crossref",
"first-page": "321",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/S0140-6736(22)02597-1_bib17",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1186/1471-2288-13-60",
"article-title": "Clinical trial simulation to evaluate power to compare the antiviral effectiveness of two hepatitis C protease inhibitors using nonlinear mixed effect models: a viral kinetic approach",
"author": "Laouénan",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/S0140-6736(22)02597-1_bib18",
"volume": "13",
"year": "2013"
},
{
"article-title": "A living WHO guideline on drugs for COVID-19",
"author": "Agarwal",
"journal-title": "BMJ",
"key": "10.1016/S0140-6736(22)02597-1_bib19",
"volume": "370",
"year": "2020"
},
{
"article-title": "Drug treatments for COVID-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"journal-title": "BMJ",
"key": "10.1016/S0140-6736(22)02597-1_bib20",
"volume": "370",
"year": "2020"
},
{
"article-title": "Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13 694 patients",
"author": "Lawrence",
"journal-title": "Research Square",
"key": "10.1016/S0140-6736(22)02597-1_bib21",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00559-2",
"article-title": "Omicron variant and booster COVID-19 vaccines",
"author": "Burki",
"doi-asserted-by": "crossref",
"first-page": "e17",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S0140-6736(22)02597-1_bib23",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.4269/ajtmh.21-1339",
"article-title": "Making statistical sense of the molnupiravir MOVe-OUT clinical trial",
"author": "Thorlund",
"doi-asserted-by": "crossref",
"first-page": "1301",
"journal-title": "Am J Trop Med Hyg",
"key": "10.1016/S0140-6736(22)02597-1_bib24",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(12)70300-6",
"article-title": "Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S0140-6736(22)02597-1_bib25",
"volume": "13",
"year": "2013"
},
{
"DOI": "10.1001/jama.293.24.3029",
"article-title": "Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "3029",
"journal-title": "JAMA",
"key": "10.1016/S0140-6736(22)02597-1_bib26",
"volume": "293",
"year": "2005"
},
{
"DOI": "10.1371/journal.pmed.1004120",
"article-title": "Favipiravir, lopinavir-ritonavir or combination therapy (FLARE): a randomised, double blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19",
"author": "Lowe",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Med",
"key": "10.1016/S0140-6736(22)02597-1_bib27",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.0030019",
"article-title": "Are racial and ethnic minorities less willing to participate in health research?",
"author": "Wendler",
"doi-asserted-by": "crossref",
"first-page": "e19",
"journal-title": "PLoS Med",
"key": "10.1016/S0140-6736(22)02597-1_bib28",
"volume": "3",
"year": "2005"
},
{
"DOI": "10.1016/j.ebiom.2021.103595",
"article-title": "The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model",
"author": "Abdelnabi",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/S0140-6736(22)02597-1_bib31",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1128/JVI.01965-17",
"article-title": "β-d-N 4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome",
"author": "Urakova",
"doi-asserted-by": "crossref",
"first-page": "e01965",
"journal-title": "J Virol",
"key": "10.1016/S0140-6736(22)02597-1_bib32",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.1136/bmjopen-2020-046799",
"article-title": "Platform randomised trial of interventions against COVID-19 in older people (PRINCIPLE): protocol for a randomised, controlled, open-label, adaptive platform, trial of community treatment of COVID-19 syndromic illness in people at higher risk",
"author": "Hayward",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/S0140-6736(22)02597-1_bib33",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00310-6",
"article-title": "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1010",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S0140-6736(22)02597-1_bib34",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"article-title": "Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial",
"author": "Dorward",
"doi-asserted-by": "crossref",
"first-page": "e446",
"journal-title": "Br J Gen Pract",
"key": "10.1016/S0140-6736(22)02597-1_bib35",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/j.ctim.2004.07.043",
"article-title": "Pragmatic clinical trials",
"author": "MacPherson",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "Complement Ther Med",
"key": "10.1016/S0140-6736(22)02597-1_bib36",
"volume": "12",
"year": "2004"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0140673622025971"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}