Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00431-6, PANORAMIC, ISRCTN30448031, Sep 2024
PRINCIPLE molnupiravir long-term followup showing improvements in time off work, healthcare use, severe symptoms, and quality of life over 6 months, however there was higher mortality (without statistical significance) and no difference in COVID-19 hospitalization, and there were significantly more new household infections in the molnupiravir group at 3-6 months.
The significantly higher household infections at 3-6 months could be related to the viral mutagenesis and persistence observed with molnupiravir, which may create variants that can evade existing immunity or other treatments.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 27.1% higher, RR 1.27, p = 0.50, treatment 20 of 11,778 (0.2%), control 15 of 11,230 (0.1%), all deaths.
|
|
risk of death, 62.1% higher, RR 1.62, p = 0.25, treatment 17 of 11,778 (0.1%), control 10 of 11,230 (0.1%), death between 29 days and 6 months.
|
|
risk of hospitalization, 7.5% lower, RR 0.93, p = 1.00, treatment 11 of 10,001 (0.1%), control 11 of 9,254 (0.1%), NNT 11263, COVID-19, 3-6 months.
|
|
relative wellness score, 1.2% better, relative time 0.99, p < 0.001, treatment mean 8.3 (±1.6) n=10,562, control mean 8.2 (±1.6) n=9,846, 3-6 months.
|
|
any severe symptom, 20.0% lower, RR 0.80, p < 0.001, treatment 10,554, control 9,840, 3-6 months.
|
|
any persistent symptom, 26.0% lower, RR 0.74, p < 0.001, treatment 9,592, control 8,634, 3-6 months.
|
|
any time off work, 25.0% lower, RR 0.75, p < 0.001, treatment 10,562, control 9,846, 3-6 months.
|
|
relative days off work, 7.7% better, RR 0.92, p = 0.52, treatment mean 1.2 (±11.1) n=10,519, control mean 1.3 (±11.1) n=9,803, 3-6 months.
|
|
new household infections, 14.0% higher, RR 1.14, p = 0.01, treatment 1,066 of 9,162 (11.6%), control 893 of 8,955 (10.0%), 3-6 months.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Harris et al., 9 Sep 2024, Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 57.0, 128 authors, study period 8 December, 2021 - 27 April, 2022, trial ISRCTN30448031 (PANORAMIC).
Contact: christopher.butler@phc.ox.
Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(24)00431-6
Background No randomised controlled trials have yet reported on the effectiveness of molnupiravir on longer term outcomes for COVID-19. The PANORAMIC trial found molnupiravir reduced time to recovery in acute COVID-19 over 28 days. We aimed to report the effect of molnupiravir treatment for COVID-19 on wellbeing, severe and persistent symptoms, new infections, health care and social service use, medication use, and time off work at 3 months and 6 months post-randomisation.
Methods This study is a follow-up to the main analysis, which was based on the first 28 days of follow-up and has been previously reported. For this multicentre, primary care, open-label, multi-arm, prospective randomised controlled trial conducted in the UK, participants were eligible if aged at least 50 years, or at least 18 years with a comorbidity, and unwell 5 days or less with confirmed COVID-19 in the community. Participants were randomly assigned to the usual care group or molnupiravir group plus usual care (800 mg twice a day for 5 days), which was stratified by age (<50 years or ≥50 years) and vaccination status (at least one dose: yes or no). The primary outcome was hospitalisation or death (or both) at 28 days; all longer term outcomes were considered to be secondary outcomes and included selfreported ratings of wellness (on a scale of 0-10), experiencing any symptom (fever, cough, shortness of breath, fatigue, muscle ache, nausea and vomiting, diarrhoea, loss of smell or taste, headache, dizziness, abdominal pain, and generally feeling unwell) rated as severe (moderately bad or major problem) or persistent, any health and social care use, health-related quality of life (measured by the EQ-5D-5L), time off work or school, new infections, and hospitalisation.
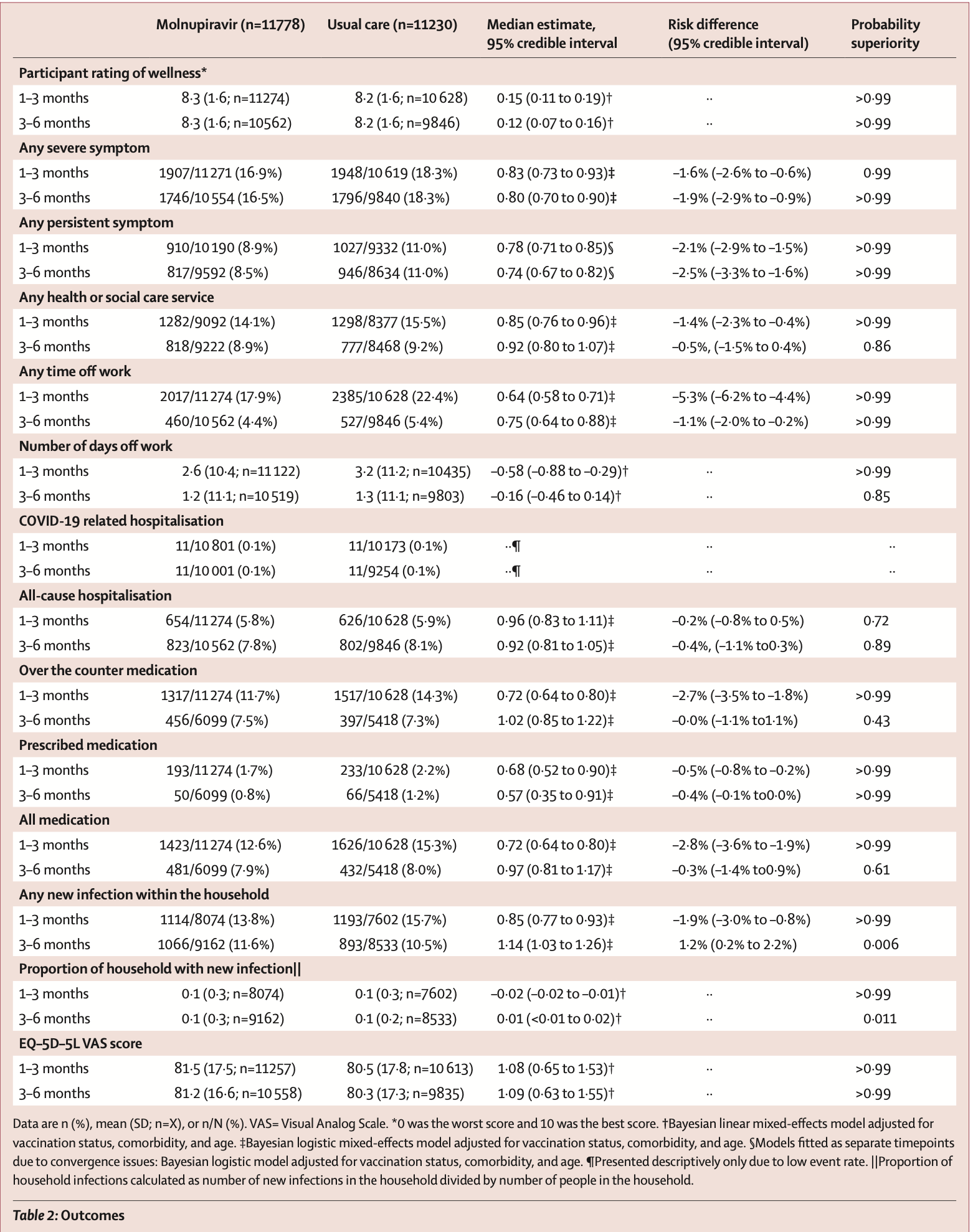
Findings Between Dec 8, 2021, and April 27, 2022, 25 783 participants were randomly assigned to the molnupiravir plus usual care group (n=12 821) or usual care group (n=12 962). Long-term follow-up data were available for 23 008 (89•2%) of 25 784 participants with 11 778 (91•9%) of 12 821 participants in the molnupiravir plus usual care group and 11 230 (86•6%) of 12 963 in the usual care group. 22 806 (99•1%) of 23 008 had at least one previous dose of a SARS-CoV-2 vaccine. Any severe (3 months: adjusted risk difference -1•6% [-2•6% to -0•6%]; probability superiority [p(sup)]>0•99; number needed to treat [NNT] 62•5; 6 months: -1•9% [-2•9% to -0•9%]; p(sup)>0•99, NNT 52•6) or persistent symptoms (3 months: adjusted risk difference -2•1% [-2•9% to -1•5%]; p(sup)>0•99; NNT 47•6; 6 months: -2•5% [-3•3% to -1•6%]; p(sup)>0•99; NNT 40) were reduced in severity, and health-related quality of life (measured by the EQ-5D-5L) improved in the molnupiravir plus usual care group at 3 months and 6 months (3 months: adjusted mean difference 1•08 [0•65 to 1•53]; p(sup)>0•99; 6 months: 1•09 [0•63 to 1•55]; p(sup)>0•99). Ratings of wellness (3 months: adjusted mean difference 0•15 (0•11 to 0•19);..
reflect best care without the drug in question, reflecting what would happen under usual circumstances. 16 The trial therefore assesses whether there is added value to adding a new drug over and above usual care. An openlabel design does not allow one to estimate the contribution of either placebo or nocebo effects to any observed differences between the randomised groups. 17, 18 Knowing whether one is taking a treatment with proven efficacy or not can affect help seeking behaviour. Subjective measures such as symptom scores and participant rating of wellbeing are at potential risk from reporting bias due to the open-label trial design. However, although for many conditions, clearly there can be substantial placebo effects for acute respiratory infections, and even where beliefs in medication are high, the estimates from open-label trials with self-report outcomes (eg, sore throat, 19 acute bronchitis, 20 and otitis 21 ) suggest either no placebo effects or minimal effects when compared with placebo controlled trials in Cochrane reviews. [22] [23] [24] [25] We have found similar evidence for COVID-19 therapeutics: the PRINCIPLE trial, using a similar open-label design, found no clinically meaningful benefit from treatment for COVID-19 with azithromycin, doxycycline, and ivermectin, [26] [27] [28] a trend for harm from treatment with colchicine, 29 and of benefit form treatment with inhaled budesionide. 30 Effect sizes in open trials are generally similar to..
References
Barsky, Saintfort, Rogers, Borus, Nonspecific medication side effects and the nocebo phenomenon, JAMA
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Butler, Dorward, Yu, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Butler, Yu, Dorward, Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, openlabel, adaptive platform trial, Lancet Respir Med
Chou, Dana, Ahmed, Long COVID models of care
Dorward, Yu, Hayward, Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract
Ford, Pragmatic trials, N Engl J Med
Fung, Baye, Baik, Mcdonald, Nirmatrelvir and molnupiravir and post-COVID-19 condition in older patients, JAMA Intern Med
Gao, Liu, Li, Xu, Zhang et al., Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials, Clin Microbiol Infect
Gbinigie, Ogburn, Allen, Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease, BMJ Open
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Hayward, Yu, Little, Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short-and longer-term outcomes, J Infection
Katz, While waiting for a randomized clinical trial of nirmatrelvir for prevention of post-COVID-19 condition, JAMA Intern Med
Little, Gould, Williamson, Moore, Warner et al., Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media, BMJ
Little, Rumsby, Kelly, Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial, JAMA
Little, Stuart, Moore, Amoxicillin for acute lower respiratory tract infection where pneumonia is not suspected clinically: a 12 country randomised placebo controlled trial in primary care, Lancet Infect Dis
Little, Williamson, Warner, Gould, Gantley et al., Open randomised trial of prescribing strategies in managing sore throat, BMJ
Moustgaard, Clayton, Jones, Impact of blinding on estimated treatment effects in randomised clinical trials: metaepidemiological study, BMJ
Schober, Bossers, Schwarte, Statistical significance versus clinical importance of observed effect sizes: what do p values and confidence intervals really represent?, Anesth Analg
Smith, Fahey, Smucny, Becker, Antibiotics for acute bronchitis, Cochrane Database Syst Rev
Spinks, Glasziou, Mar, Antibiotics for treatment of sore throat in children and adults, Cochrane Database Syst Rev
Standing, Buggiotti, Guerra-Assuncao, Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nat Commun
Thorpe, Zwarenstein, Oxman, A pragmaticexplanatory continuum indicator summary (PRECIS): a tool to help trial designers, J Clin Epidemiol
Venekamp, Sanders, Glasziou, Rovers, Antibiotics for acute otitis media in children, Cochrane Database Syst Rev
Wartolowska, The nocebo effect as a source of bias in the assessment of treatment effects, Res
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, BMJ
Yang, Tebbutt, Long COVID: the next public health crisis is already on its way, Lancet Reg Health Eur
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
Zuidgeest, Goetz, Grobbee, PRECIS-2 in perspective: what is next for pragmatic trials?, J Clin Epidemiol
DOI record:
{
"DOI": "10.1016/s1473-3099(24)00431-6",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(24)00431-6",
"alternative-id": [
"S1473309924004316"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(24)00431-6"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(24)00436-5"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Harris",
"given": "Victoria",
"sequence": "first"
},
{
"affiliation": [],
"family": "Holmes",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gbinigie-Thompson",
"given": "Oghenekome",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Najib M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richards",
"given": "Duncan B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayward",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dorward",
"given": "Jienchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lowe",
"given": "David M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Standing",
"given": "Joseph F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breuer",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khoo",
"given": "Saye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petrou",
"given": "Stavros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hood",
"given": "Kerenza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Haroon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carson-Stevens",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen-Van-Tam",
"given": "Jonathan S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahendra G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saville",
"given": "Benjamin R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francis",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Nicholas P B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dobson",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Png",
"given": "May Ee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lown",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Hecke",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jani",
"given": "Bhautesh D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hart",
"given": "Nigel D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cureton",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patil",
"given": "Meena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andersson",
"given": "Monique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coates",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bateman",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Jennifer C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raymundo-Wood",
"given": "Ivy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Ly-Mee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hobbs",
"given": "F D Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Little",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Christopher C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moftah",
"given": "Areej",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halifax",
"given": "Rob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turnbull",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sundaralingam",
"given": "Anand",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agyeman",
"given": "Akosua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Julianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thalasselis",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woodall",
"given": "Maximillian N J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yongblah",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Howell",
"given": "Aleksandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Kavil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussain",
"given": "Iqbal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Penfold",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutchinson",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poonian",
"given": "Satveer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imlach",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Popoola",
"given": "Olajide",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Irving",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pora",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobsen",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Vibhore",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kennard",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Rishabh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Umar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Razzaq",
"given": "Omair",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knox",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Scot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krasucki",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Royal",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Law",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Safa",
"given": "Afsana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Rem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sehdev",
"given": "Satash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lester",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sevenoaks",
"given": "Tamsin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheikh",
"given": "Aadil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lunn",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Short",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mackintosh",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh Sidhu",
"given": "Baljinder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathukia",
"given": "Mehul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Ivor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soni",
"given": "Yusuf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morton",
"given": "Seb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "Pete",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wingfield",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nally",
"given": "Rhiannon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ndukauba",
"given": "Chinonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wooding",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogundapo",
"given": "Olufunto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woods",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okeke",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yong",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Tanveer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allcock",
"given": "Damien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atherton",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beltran-Martinez",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benedict",
"given": "Oluseye Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bird",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brennan",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burns",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carson-Stevens",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Zelda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danson",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Kare-Silver",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhasmana",
"given": "Devesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dickson",
"given": "Jon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engamba",
"given": "Serge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fisher",
"given": "Stacey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frost",
"given": "Eve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaunt",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghosh",
"given": "Sarit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilkar",
"given": "Ishtiaq",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Granier",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Packham",
"given": "Alice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dowsell",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gulati",
"given": "Radhika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Amit",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
9
]
],
"date-time": "2024-09-09T22:34:21Z",
"timestamp": 1725921261000
},
"deposited": {
"date-parts": [
[
2024,
9,
9
]
],
"date-time": "2024-09-09T22:34:35Z",
"timestamp": 1725921275000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "NIHR"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
10
]
],
"date-time": "2024-09-10T00:27:01Z",
"timestamp": 1725928021591
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
31
]
],
"date-time": "2024-07-31T00:00:00Z",
"timestamp": 1722384000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924004316?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924004316?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
9
]
]
},
"published-print": {
"date-parts": [
[
2024,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Long COVID: the next public health crisis is already on its way",
"author": "Yang",
"journal-title": "Lancet Reg Health Eur",
"key": "10.1016/S1473-3099(24)00431-6_bib1",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2023.04.014",
"article-title": "Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "979",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/S1473-3099(24)00431-6_bib4",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00431-6_bib5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(24)00431-6_bib6",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1038/s41467-024-45641-0",
"article-title": "Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients",
"author": "Standing",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S1473-3099(24)00431-6_bib7",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00431-6_bib8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2023.5099",
"article-title": "Nirmatrelvir and molnupiravir and post-COVID-19 condition in older patients",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "1404",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S1473-3099(24)00431-6_bib9",
"volume": "183",
"year": "2023"
},
{
"article-title": "Molnupiravir and risk of post-acute sequelae of covid-19: cohort study",
"author": "Xie",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00431-6_bib10",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2023.0760",
"article-title": "While waiting for a randomized clinical trial of nirmatrelvir for prevention of post-COVID-19 condition",
"author": "Katz",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S1473-3099(24)00431-6_bib11",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1136/bmjopen-2022-069176",
"author": "Gbinigie",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/S1473-3099(24)00431-6_bib12",
"volume": "13",
"year": "2023"
},
{
"author": "Chou",
"key": "10.1016/S1473-3099(24)00431-6_bib13",
"series-title": "Long COVID models of care",
"year": "2024"
},
{
"DOI": "10.1016/j.jclinepi.2008.12.011",
"article-title": "A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers",
"author": "Thorpe",
"doi-asserted-by": "crossref",
"first-page": "464",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/S1473-3099(24)00431-6_bib14",
"volume": "62",
"year": "2009"
},
{
"DOI": "10.1016/j.jclinepi.2016.02.027",
"article-title": "PRECIS-2 in perspective: what is next for pragmatic trials?",
"author": "Zuidgeest",
"doi-asserted-by": "crossref",
"first-page": "22",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/S1473-3099(24)00431-6_bib15",
"volume": "84",
"year": "2017"
},
{
"DOI": "10.1056/NEJMra1510059",
"article-title": "Pragmatic trials",
"author": "Ford",
"doi-asserted-by": "crossref",
"first-page": "454",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00431-6_bib16",
"volume": "375",
"year": "2016"
},
{
"DOI": "10.1001/jama.287.5.622",
"article-title": "Nonspecific medication side effects and the nocebo phenomenon",
"author": "Barsky",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(24)00431-6_bib17",
"volume": "287",
"year": "2002"
},
{
"article-title": "The nocebo effect as a source of bias in the assessment of treatment effects",
"author": "Wartolowska",
"first-page": "5",
"journal-title": "F1000 Res",
"key": "10.1016/S1473-3099(24)00431-6_bib18",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1136/bmj.314.7082.722",
"article-title": "Open randomised trial of prescribing strategies in managing sore throat",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "722",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00431-6_bib19",
"volume": "314",
"year": "1997"
},
{
"DOI": "10.1001/jama.293.24.3029",
"article-title": "Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "3029",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(24)00431-6_bib20",
"volume": "293",
"year": "2005"
},
{
"DOI": "10.1136/bmj.322.7282.336",
"article-title": "Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "336",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00431-6_bib21",
"volume": "322",
"year": "2001"
},
{
"article-title": "Antibiotics for treatment of sore throat in children and adults",
"author": "Spinks",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/S1473-3099(24)00431-6_bib22",
"volume": "12",
"year": "2021"
},
{
"article-title": "Antibiotics for acute bronchitis",
"author": "Smith",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/S1473-3099(24)00431-6_bib23",
"volume": "2017",
"year": "2017"
},
{
"article-title": "Antibiotics for acute otitis media in children",
"author": "Venekamp",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/S1473-3099(24)00431-6_bib24",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(12)70300-6",
"article-title": "Amoxicillin for acute lower respiratory tract infection where pneumonia is not suspected clinically: a 12 country randomised placebo controlled trial in primary care",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(24)00431-6_bib25",
"volume": "13",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(21)00461-X",
"article-title": "Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(24)00431-6_bib26",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00310-6",
"article-title": "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1010",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S1473-3099(24)00431-6_bib27",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2024.106130",
"article-title": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"author": "Hayward",
"doi-asserted-by": "crossref",
"journal-title": "J Infection",
"key": "10.1016/S1473-3099(24)00431-6_bib28",
"volume": "88",
"year": "2024"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"article-title": "Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial",
"author": "Dorward",
"doi-asserted-by": "crossref",
"first-page": "e446",
"journal-title": "Br J Gen Pract",
"key": "10.1016/S1473-3099(24)00431-6_bib29",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(24)00431-6_bib30",
"volume": "398",
"year": "2021"
},
{
"article-title": "Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study",
"author": "Moustgaard",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00431-6_bib31",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1213/ANE.0000000000002798",
"article-title": "Statistical significance versus clinical importance of observed effect sizes: what do p values and confidence intervals really represent?",
"author": "Schober",
"doi-asserted-by": "crossref",
"first-page": "1068",
"journal-title": "Anesth Analg",
"key": "10.1016/S1473-3099(24)00431-6_bib32",
"volume": "126",
"year": "2018"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309924004316"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}