Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(22)00644-2, AGILE CST-2, NCT04746183, Jul 2022 (preprint)
RCT 90 molnupiravir and 90 placebo patients, showing faster viral clearance with treatment, not reaching the pre-defined threshold for superiority and recommendation as a candidate for large scale evaluation.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of oxygen therapy, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 90 (0.0%), control 1 of 90 (1.1%), NNT 90, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 88.9% lower, RR 0.11, p = 0.12, treatment 0 of 90 (0.0%), control 4 of 90 (4.4%), NNT 22, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 12.5% higher, RR 1.12, p = 0.74, treatment 27 of 90 (30.0%), control 24 of 90 (26.7%), day 29, Table S2.
|
|
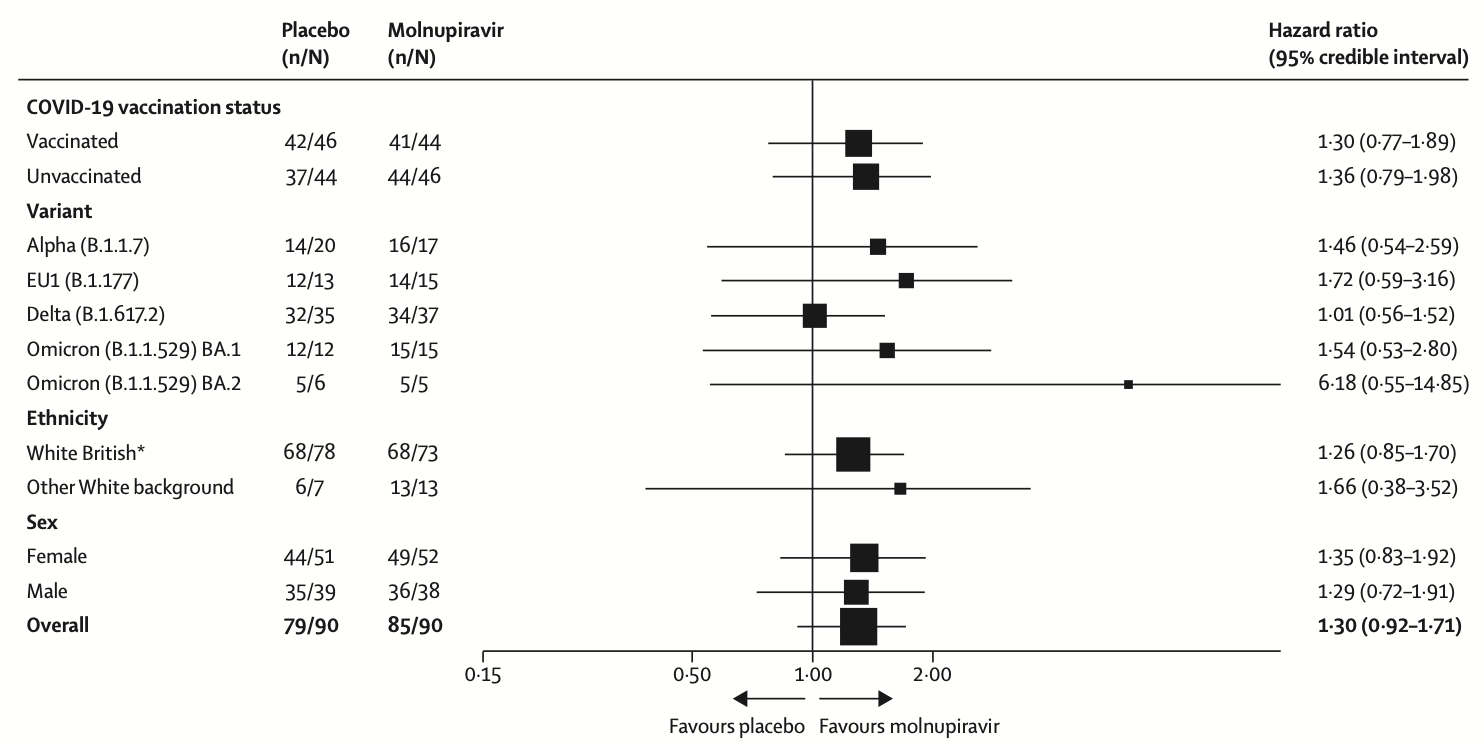
risk of no viral clearance, 23.1% lower, HR 0.77, p = 0.07, treatment 90, control 90, inverted to make HR<1 favor treatment, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Khoo et al., 24 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, median age 43.0, 90 authors, study period 18 November, 2020 - 16 March, 2022, average treatment delay 3.3 days, trial NCT04746183 (history) (AGILE CST-2).
Contact: khoo@liverpool.ac.uk.
Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(22)00644-2
Background The antiviral drug molnupiravir was licensed for treating at-risk patients with COVID-19 on the basis of data from unvaccinated adults. We aimed to evaluate the safety and virological efficacy of molnupiravir in vaccinated and unvaccinated individuals with COVID-19. Methods This randomised, placebo-controlled, double-blind, phase 2 trial (AGILE CST-2) was done at five National Institute for Health and Care Research sites in the UK. Eligible participants were adult (aged ≥18 years) outpatients with PCR-confirmed, mild-to-moderate SARS-CoV-2 infection who were within 5 days of symptom onset. Using permuted blocks (block size 2 or 4) and stratifying by site, participants were randomly assigned (1:1) to receive either molnupiravir (orally; 800 mg twice daily for 5 days) plus standard of care or matching placebo plus standard of care.
The primary outcome was the time from randomisation to SARS-CoV-2 PCR negativity on nasopharyngeal swabs and was analysed by use of a Bayesian Cox proportional hazards model for estimating the probability of a superior virological response (hazard ratio [HR]>1 ) for molnupiravir versus placebo. Our primary model used a two-point prior based on equal prior probabilities (50%) that the HR was 1•0 or 1•5. We defined a priori that if the probability of a HR of more than 1 was more than 80% molnupiravir would be recommended for further testing. The primary outcome was analysed in the intention-to-treat population and safety was analysed in the safety population, comprising participants who had received at least one dose of allocated treatment. This trial is registered in ClinicalTrials.gov, NCT04746183, and the ISRCTN registry, ISRCTN27106947, and is ongoing.
(199 grade 1-2 and one grade ≥3) in the molnupiravir group and 219 adverse events (211 grade 1-2 and eight grade ≥3) in the placebo group. One participant in the molnupiravir group (grade 3 hypertension) and three participants in the placebo group (eight events; grade 3
Discussion Molnupiravir received conditional marketing authorisation from the UK Medicines and Healthcare products Regulatory Agency and early use authorisation from the US Food and Drug Administration on the basis of data from the MOVe-OUT study 2 in unvaccinated individuals at high risk of severe disease who were infected with the SARS-CoV-2 variants in circulation between May and October, 2021. 2 MOVe-OUT found that molnupiravir had good tolerability and reduced the number of hospitalisations and deaths by about 50% at the interim evaluation, falling to around 30% after all 1433 patients had been analysed. An evaluation of the effect of molnupiravir on virological response by SARS-CoV-2 variant or in vaccinated patients within a randomised controlled trial has not been previously published. In our phase 2 study, patients in the molnupiravir group had a faster median time from randomisation to PCR negativity than did patients in the placebo group. We used a Bayesian framework to facilitate decision making. Using a two-point prior approach, the probability of the HR for PCR negativity being more than 1 (ie, in favour of molnupiravir vs placebo) was 75•4%, which was less than the 80% threshold we had set..
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Coolen, Wolters, Tostmann, SARS-CoV-2 wholegenome sequencing using reverse complement PCR: for easy, fast and accurate outbreak and variant analysis, J Clin Virol
Ewings, Saunders, Jaki, Mozgunov, Practical recommendations for implementing a Bayesian adaptive phase I design during a pandemic, BMC Med Res Methodol
Griffiths, Fitzgerald, Jaki, AGILE: a seamless phase I/ IIa platform for the rapid evaluation of candidates for COVID-19 treatment: an update to the structured summary of a study protocol for a randomised platform trial letter, Lancet Infect Dis
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med
Khoo, Fitzgerald, Fletcher, Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase I, openlabel, dose-escalating, randomized controlled study, J Antimicrob Chemother
Kumarasamy, Saha, Jindal, Phase 3 trial of molnupiravir in adults with mild SARS-CoV-2 infection in India, Conference on Retroviruses and Opportunistic Infections
O'toole, Scher, Underwood, Assignment of epidemiological lineages in an emerging pandemic using the Pangolin tool, Virus Evol
Troth, Butterton, Deanda, Letter to the editor in response to Zhou et al, J Infect Dis
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00507-2
DOI record:
{
"DOI": "10.1016/s1473-3099(22)00644-2",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(22)00644-2",
"alternative-id": [
"S1473309922006442"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(22)00644-2"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(22)00665-X"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Khoo",
"given": "Saye H",
"sequence": "first"
},
{
"affiliation": [],
"family": "FitzGerald",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Geoffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Middleton",
"given": "Calley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmad",
"given": "Shazaad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Christopher J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadjiyiannakis",
"given": "Dennis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyon",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaw",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mozgunov",
"given": "Pavel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Periselneris",
"given": "Jimstan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woods",
"given": "Christie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bullock",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hale",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reynolds",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Downs",
"given": "Nichola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ewings",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buadi",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cameron",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knox",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donovan-Banfield",
"given": "I'ah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenhalf",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiong",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavelle-Langham",
"given": "Lara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Northey",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Painter",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holman",
"given": "Wayne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lalloo",
"given": "David G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tetlow",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiscox",
"given": "Julian A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fletcher",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paton",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayden",
"given": "Fred",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darbyshire",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucas",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorch",
"given": "Ulrika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freedman",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knight",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Julious",
"given": "Stevan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Byrne",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cubas Atienzar",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Jayne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dixon",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alexandersson",
"given": "Anja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hatchard",
"given": "Parys",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tilt",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Titman",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doce Carracedo",
"given": "Ale",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chandran Gorner",
"given": "Vatsi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woodhouse",
"given": "Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carlucci",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okenyi",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bula",
"given": "Marcin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dodd",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibney",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dry",
"given": "Lesley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rashid Gardner",
"given": "Zalina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sammour",
"given": "Amin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cole",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rowland",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsakiroglu",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yip",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osanlou",
"given": "Rostam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stewart",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parker",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turgut",
"given": "Tolga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Afshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Starkey",
"given": "Kay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Subin",
"given": "Sujamole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stockdale",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herring",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Jonathon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliver",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pacurar",
"given": "Mihaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owens",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Munro",
"given": "Alistair",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babbage",
"given": "Gavin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faust",
"given": "Saul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harvey",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pratt",
"given": "Danny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagra",
"given": "Deepak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vyas",
"given": "Aashish",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T23:06:23Z",
"timestamp": 1666220783000
},
"deposited": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T23:06:38Z",
"timestamp": 1666220798000
},
"indexed": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T23:42:25Z",
"timestamp": 1666222945780
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
13
]
],
"date-time": "2022-09-13T00:00:00Z",
"timestamp": 1663027200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309922006442?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309922006442?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1093/jac/dkab318",
"article-title": "Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a phase I, open-label, dose-escalating, randomized controlled study",
"author": "Khoo",
"doi-asserted-by": "crossref",
"first-page": "3286",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S1473-3099(22)00644-2_bib1",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(22)00644-2_bib2",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/S1473-3099(22)00644-2_bib3",
"unstructured": "Kumarasamy N, Saha B, Jindal A, et al. Phase 3 trial of molnupiravir in adults with mild SARS-CoV-2 infection in India. Conference on Retroviruses and Opportunistic Infections; Feb 15, 2022 (abstract O-9)."
},
{
"DOI": "10.1093/infdis/jiab362",
"article-title": "Letter to the editor in response to Zhou et al",
"author": "Troth",
"doi-asserted-by": "crossref",
"first-page": "1442",
"journal-title": "J Infect Dis",
"key": "10.1016/S1473-3099(22)00644-2_bib4",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1186/s13063-021-05458-4",
"article-title": "AGILE: a seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment: an update to the structured summary of a study protocol for a randomised platform trial letter",
"author": "Griffiths",
"doi-asserted-by": "crossref",
"first-page": "487",
"journal-title": "Trials",
"key": "10.1016/S1473-3099(22)00644-2_bib6",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(22)00644-2_bib7",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2021.104993",
"article-title": "SARS-CoV-2 whole-genome sequencing using reverse complement PCR: for easy, fast and accurate outbreak and variant analysis",
"author": "Coolen",
"doi-asserted-by": "crossref",
"first-page": "104993",
"journal-title": "J Clin Virol",
"key": "10.1016/S1473-3099(22)00644-2_bib8",
"volume": "144",
"year": "2021"
},
{
"DOI": "10.1093/ve/veab064",
"article-title": "Assignment of epidemiological lineages in an emerging pandemic using the Pangolin tool",
"author": "O'Toole",
"doi-asserted-by": "crossref",
"first-page": "veab064",
"journal-title": "Virus Evol",
"key": "10.1016/S1473-3099(22)00644-2_bib9",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1186/s12874-022-01512-0",
"article-title": "Practical recommendations for implementing a Bayesian adaptive phase I design during a pandemic",
"author": "Ewings",
"doi-asserted-by": "crossref",
"first-page": "25",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/S1473-3099(22)00644-2_bib10",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Nat Med",
"key": "10.1016/S1473-3099(22)00644-2_bib11",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(22)00644-2_bib13",
"year": "2022"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309922006442"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}