Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-024-01010-4, Jun 2024
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
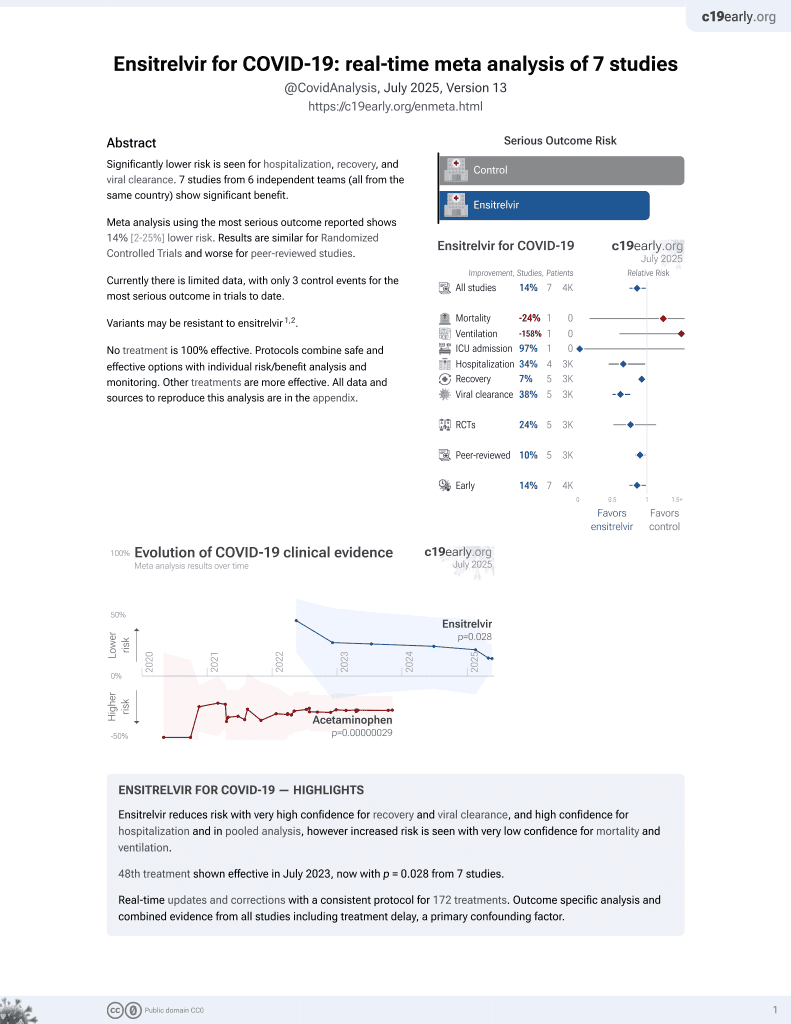
Retrospective 167,310 high-risk COVID-19 outpatients in Japan showing significantly lower hospitalization with ensitrelvir treatment.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 23.7% higher, RR 1.24, p = 0.84, treatment 5,177, control 162,133, propensity score weighting.
|
|
risk of mechanical ventilation, 158.5% higher, RR 2.58, p = 0.20, treatment 5,177, control 162,133, propensity score weighting.
|
|
risk of ICU admission, 97.3% lower, RR 0.03, p = 0.63, treatment 0 of 5,177 (0.0%), control 35 of 162,133 (0.0%), NNT 4632, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 36.5% lower, RR 0.64, p = 0.33, treatment 5,177, control 162,133, propensity score weighting.
|
|
risk of hospitalization, 37.1% lower, RR 0.63, p = 0.02, treatment 5,177, control 162,133, propensity score weighting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Takazono et al., 28 Jun 2024, retrospective, Japan, peer-reviewed, 13 authors, study period November 2022 - July 2023.
Contact: satoki.fujita@shionogi.co.jp.
Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19
Infectious Diseases and Therapy, doi:10.1007/s40121-024-01010-4
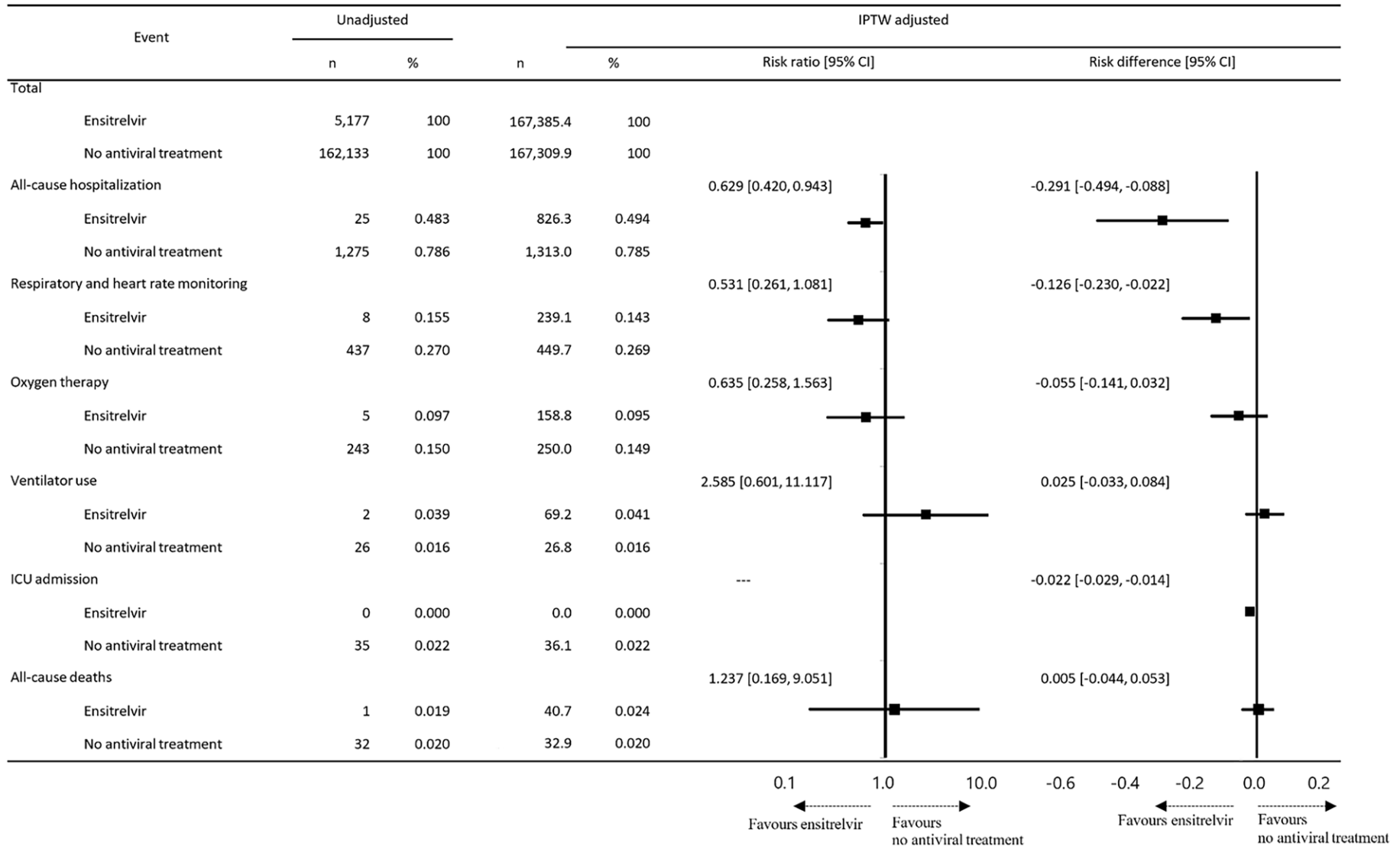
Introduction: This study aimed to evaluate the effectiveness of ensitrelvir, an oral antiviral, in reducing hospitalization risk in outpatients at high-risk for severe COVID-19 during the Omicron era. Methods: This was a retrospective study using a large Japanese health insurance claims database. It included high-risk outpatients for severe symptoms who received their first COVID-19 diagnosis between November 2022 and July 2023. The study included outpatients aged ≥ 18 years. The primary endpoint was all-cause hospitalization during the 4-week period from the date of outpatient diagnosis and medication, comparing the ensitrelvir group (n = 5177) and the no antiviral treatment group (n = 162,133). The risk ratio and risk difference were evaluated after adjusting patient background distribution by the inverse probability of treatment weight (IPTW) method. Secondary endpoints were incidence of respiratory and heart rate monitoring, oxygen therapy, ventilator use, intensive care admission, and all-cause death.
Results: The risk ratio for all-cause hospitalization between the ensitrelvir group (n = 167,385) and the no antiviral treatment group (n = 167,310) after IPTW adjustment was 0.629 [95% confidence interval (CI) 0.420, 0.943]. The risk difference was -0.291 [95% CI -0.494, -0.088]. The incidence of both respiratory and
Open Access. This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by-nc/4. 0/ . Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Clinicaltrials, Gov, Strategies and treatments for respiratory infections & viral emergencies (STRIVE): Shionogi protease inhibitor
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Ison, Portsmouth, Yoshida, Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial, Lancet Infect Dis
Kumamaru, Togo, Kimura, Inventory of real-world data sources in Japan: Annual survey conducted by the Japanese Society for Pharmacoepidemiology Task Force, Pharmacoepidemiol Drug Saf
Kuroda, Nobori, Fukao, Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo, J Antimicrob Chemother
Madhi, Kwatra, Myers, Population immunity and Covid-19 severity with Omicron variant in South Africa, N Engl J Med
Manchanda, Mitra, Rafique, Is Omicron really mild?-comparative analysis of comorbidities and disease outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants, Indian J Med Microbiol
Miyauchi, Komeda, Fujiwara, Comparison of hospitalization and death frequencies in influenza outpatients treated with baloxavir marboxil or neuraminidase inhibitors: an observational database study, Jpn J Pharmacoepidemiol
Oshitani, Komeda, Fujita, Asakawa, Kitanishi, Comparison of the incidence of severe outcomes in outpatients with COVID-19 or seasonal influenza without risk factors: retrospective analysis of a health insurance claims-database, J Clin Virol Plus
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September 2022, MMWR Morb Mortal Wkly Rep
Shim, Chan, Owens, Jaffe, Prentice et al., Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: a systematic review and meta-analysis, Health Sci Rep
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant
Yamato, Kinoshita, Miyazawa, Seki, Mizuno et al., Ensitrelvir in patients with SARS-CoV-2: a retrospective chart review, J Infect Chemother
DOI record:
{
"DOI": "10.1007/s40121-024-01010-4",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-024-01010-4",
"alternative-id": [
"1010"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "29 April 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 June 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 June 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Satoki Fujita, Takuji Komeda, Shogo Miyazawa, Yuki Yoshida, Yoshitake Kitanishi, Masahiro Kinoshita, Satoshi Kojima, Huilian Shen, and Takeki Uehara are employees of Shionogi & Co., Ltd. and may hold stocks in the company. Takahiro Takazono has received personal fees from Shionogi & Co., Ltd., MSD K.K., Pfizer Japan Inc., Insmed GK., Asahi Kasei Pharma Corporation, and Kyorin Pharmaceutical Co., Ltd. Hiroshi Mukae has also received personal fees from AbbVie GK., Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AstraZeneca K.K., Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., FUJIFILM Toyama Chemical Co., Ltd., Gilead Sciences Inc., Insmed GK., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nihon Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Sumitomo Pharma Co., Ltd., Taiho Pharmaceutical Co., Ltd., Taisho Pharma Co., Ltd., Teijin Healthcare Ltd., and Toa Shinyaku Co., Ltd., and grants from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., FUJIFILM Toyama Chemical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Toa Shinyaku Co., Ltd., and Torii Pharmaceutical Co., Ltd., outside the submitted work. Naoki Hosogaya and Naoki Iwanaga have no conflicts of interest to disclose."
},
{
"group": {
"label": "Ethical Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This study was conducted according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects. It used anonymized information from an existing database, and informed consent was therefore not required. This study was registered in UMIN Clinical Trials Registry (study ID: UMIN000053217)."
}
],
"author": [
{
"affiliation": [],
"family": "Takazono",
"given": "Takahiro",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fujita",
"given": "Satoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Komeda",
"given": "Takuji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miyazawa",
"given": "Shogo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoshida",
"given": "Yuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kitanishi",
"given": "Yoshitake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinoshita",
"given": "Masahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kojima",
"given": "Satoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Huilian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uehara",
"given": "Takeki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hosogaya",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iwanaga",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mukae",
"given": "Hiroshi",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
28
]
],
"date-time": "2024-06-28T04:03:28Z",
"timestamp": 1719547408000
},
"deposited": {
"date-parts": [
[
2024,
7,
23
]
],
"date-time": "2024-07-23T17:06:34Z",
"timestamp": 1721754394000
},
"funder": [
{
"DOI": "10.13039/501100005612",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100005612",
"id-type": "DOI"
}
],
"name": "Shionogi"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
6
]
],
"date-time": "2025-05-06T13:31:21Z",
"timestamp": 1746538281355,
"version": "3.37.3"
},
"is-referenced-by-count": 11,
"issue": "8",
"issued": {
"date-parts": [
[
2024,
6,
28
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2024,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
28
]
],
"date-time": "2024-06-28T00:00:00Z",
"timestamp": 1719532800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
28
]
],
"date-time": "2024-06-28T00:00:00Z",
"timestamp": 1719532800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-01010-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-024-01010-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-024-01010-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "1821-1833",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2024,
6,
28
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
28
]
]
},
"published-print": {
"date-parts": [
[
2024,
8
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.ijmmb.2023.100391",
"author": "V Manchanda",
"doi-asserted-by": "publisher",
"journal-title": "Indian J Med Microbiol",
"key": "1010_CR1",
"unstructured": "Manchanda V, Mitra S, Rafique I, et al. Is Omicron really mild?—comparative analysis of comorbidities and disease outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants. Indian J Med Microbiol. 2023;45: 100391.",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "1010_CR2",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "1010_CR3",
"unstructured": "Jayk Bernal A, da Silva MMG, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "1010_CR4",
"unstructured": "Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022;387(9):790–8.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7148e2",
"author": "MM Shah",
"doi-asserted-by": "publisher",
"first-page": "1531",
"issue": "48",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "1010_CR5",
"unstructured": "Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19–United States, April–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531–7.",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "1010_CR6",
"unstructured": "Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342–9.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "1010_CR7",
"unstructured": "Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–93.",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1007/s10238-022-00949-3",
"author": "Y Suzuki",
"doi-asserted-by": "publisher",
"first-page": "2715",
"issue": "6",
"journal-title": "Clin Exp Med",
"key": "1010_CR8",
"unstructured": "Suzuki Y, Shibata Y, Minemura H, et al. Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic. Clin Exp Med. 2023;23(6):2715–23.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "1010_CR9",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–22.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"author": "H Yotsuyanagi",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "JAMA Netw Open",
"key": "1010_CR10",
"unstructured": "Yotsuyanagi H, Ohmagari N, Doi Y, et al. Efficacy and safety of 5-day oral ensitrelvir for patients with mild-to-moderate COVID-19: the SCORPIO-SR randomized clinical trial. JAMA Netw Open. 2024;7(2): e2354991.",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciac933",
"author": "H Mukae",
"doi-asserted-by": "publisher",
"first-page": "1403",
"issue": "8",
"journal-title": "Clin Infect Dis",
"key": "1010_CR11",
"unstructured": "Mukae H, Yotsuyanagi H, Ohmagari N, et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76(8):1403–11.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1128/aac.00697-22",
"author": "H Mukae",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "Antimicrob Agents Chemother",
"key": "1010_CR12",
"unstructured": "Mukae H, Yotsuyanagi H, Ohmagari N, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66(10): e0069722.",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1016/j.jiac.2023.09.008",
"author": "I Sakamaki",
"doi-asserted-by": "publisher",
"first-page": "147",
"issue": "2",
"journal-title": "J Infect Chemother",
"key": "1010_CR13",
"unstructured": "Sakamaki I, Negoro E, Iwasaki H, Yamauchi T. Ensitrelvir eradicates persistent SARS-CoV-2 infection in a follicular lymphoma patient treated with anti-CD20 antibodies. J Infect Chemother. 2024;30(2):147–9.",
"volume": "30",
"year": "2024"
},
{
"author": "A Fujimoto",
"first-page": "1036",
"issue": "12",
"journal-title": "BIO Clin",
"key": "1010_CR14",
"unstructured": "Fujimoto A, Shibata K, Miyazawa S, Sonoyama T. Clinical outcomes after administration of ensitrelvir fumaric acid in nonhospitalized patients with COVID-19: a single center, retrospective observational study. BIO Clin. 2023;38(12):1036–42 (in Japanese).",
"volume": "38",
"year": "2023"
},
{
"author": "M Konishi",
"first-page": "32",
"issue": "2",
"journal-title": "J Kyoto Med Assoc",
"key": "1010_CR15",
"unstructured": "Konishi M. Examination of the pathological condition of outpatient patients with fever during the 8th wave of the new coronavirus infection (COVID-19): evaluation of prognosis and sequelae due to oral antiviral drug administration. J Kyoto Med Assoc. 2023;70(2):32–9 (in Japanese).",
"volume": "70",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2024.1287300",
"author": "C Furuya",
"doi-asserted-by": "publisher",
"first-page": "1287300",
"journal-title": "Front Immunol",
"key": "1010_CR16",
"unstructured": "Furuya C, Yasuda H, Hiki M, et al. Case report: Ensitrelvir for treatment of persistent COVID-19 in lymphoma patients: a report of two cases. Front Immunol. 2024;15:1287300.",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.jiac.2024.02.015",
"doi-asserted-by": "crossref",
"key": "1010_CR17",
"unstructured": "Yamato M, Kinoshita M, Miyazawa S, Seki M, Mizuno T, Sonoyama T. Ensitrelvir in patients with SARS-CoV-2: a retrospective chart review. J Infect Chemother. 2024 (in press)."
},
{
"key": "1010_CR18",
"unstructured": "Ministry of Health, Labour and Welfare. Version 8.1 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/000997789.pdf. Accessed May 30, 2024 (in Japanese)."
},
{
"key": "1010_CR19",
"unstructured": "Ministry of Health, Labour and Welfare. Version 9 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/000936655.pdf. Accessed May 30, 2024 (in Japanese)."
},
{
"key": "1010_CR20",
"unstructured": "Ministry of Health, Labour and Welfare. Version 10 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/001136687.pdf. Accessed May 30, 2024 (in Japanese)."
},
{
"DOI": "10.1016/j.jcvp.2023.100175",
"author": "H Oshitani",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Clin Virol Plus",
"key": "1010_CR21",
"unstructured": "Oshitani H, Komeda T, Fujita S, Asakawa M, Kitanishi Y. Comparison of the incidence of severe outcomes in outpatients with COVID-19 or seasonal influenza without risk factors: retrospective analysis of a health insurance claims-database. J Clin Virol Plus. 2024;4(1): 100175.",
"volume": "4",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2119658",
"author": "SA Madhi",
"doi-asserted-by": "publisher",
"first-page": "1314",
"issue": "14",
"journal-title": "N Engl J Med",
"key": "1010_CR22",
"unstructured": "Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386(14):1314–26.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(20)30004-9",
"author": "MG Ison",
"doi-asserted-by": "publisher",
"first-page": "1204",
"issue": "10",
"journal-title": "Lancet Infect Dis",
"key": "1010_CR23",
"unstructured": "Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–14.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1002/hsr2.241",
"author": "SJ Shim",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Health Sci Rep",
"key": "1010_CR24",
"unstructured": "Shim SJ, Chan M, Owens L, Jaffe A, Prentice B, Homaira N. Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: a systematic review and meta-analysis. Health Sci Rep. 2021;4(1): e241.",
"volume": "4",
"year": "2021"
},
{
"author": "H Miyauchi",
"first-page": "15",
"issue": "1",
"journal-title": "Jpn J Pharmacoepidemiol",
"key": "1010_CR25",
"unstructured": "Miyauchi H, Komeda T, Fujiwara M, et al. Comparison of hospitalization and death frequencies in influenza outpatients treated with baloxavir marboxil or neuraminidase inhibitors: an observational database study. Jpn J Pharmacoepidemiol. 2021;26(1):15–26 (in Japanese).",
"volume": "26",
"year": "2021"
},
{
"author": "O Puhach",
"first-page": "147",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "1010_CR26",
"unstructured": "Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023;21(3):147–61.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkad027",
"author": "T Kuroda",
"doi-asserted-by": "publisher",
"first-page": "946",
"issue": "4",
"journal-title": "J Antimicrob Chemother",
"key": "1010_CR27",
"unstructured": "Kuroda T, Nobori H, Fukao K, et al. Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo. J Antimicrob Chemother. 2023;78(4):946–52.",
"volume": "78",
"year": "2023"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"author": "HK Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"issue": "5",
"journal-title": "J Heart Lung Transplant",
"key": "1010_CR28",
"unstructured": "Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–7.",
"volume": "39",
"year": "2020"
},
{
"key": "1010_CR29",
"unstructured": "Ministry of Health, Labour and Welfare. D220 Respiratory Heart Rate Monitoring, Neonatal Heart Rate and Respiratory Monitoring, Cardioscope (Heart Scope). Notification from the Insurance Bureau. Implementation Considerations for the Partial Revision of the Calculation Method for Medical Reimbursement. 2022; No.0304-1, Mar 4: 346–347. https://kouseikyoku.mhlw.go.jp/kyushu/000215073.pdf. Accessed May 30, 2024 (in Japanese)."
},
{
"key": "1010_CR30",
"unstructured": "Ministry of Health, Labour and Welfare. Number of newly confirmed cases by age (weekly). Visualizing the data: information on COVID-19 infections. https://covid19.mhlw.go.jp/en/. Accessed May 30, 2024 (in Japanese)."
},
{
"key": "1010_CR31",
"unstructured": "ClinicalTrials.gov. Strategies and treatments for respiratory infections & viral emergencies (STRIVE): Shionogi protease inhibitor. NCT05605093. https://clinicaltrials.gov/study/NCT05605093. Accessed May 30, 2024."
},
{
"key": "1010_CR32",
"unstructured": "Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed May 30, 2024."
},
{
"DOI": "10.1002/pds.5680",
"author": "H Kumamaru",
"doi-asserted-by": "publisher",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "1010_CR33",
"unstructured": "Kumamaru H, Togo K, Kimura T, et al. Inventory of real-world data sources in Japan: Annual survey conducted by the Japanese Society for Pharmacoepidemiology Task Force. Pharmacoepidemiol Drug Saf. 2024;33: e5680.",
"volume": "33",
"year": "2024"
},
{
"key": "1010_CR34",
"unstructured": "Ministry of Health, Labor and Welfare. Document 2–5: The 98th COVID-19 Countermeasures Advisory Board Meeting of the Ministry of Health, Labor and Welfare (September 7, 2022).. Accessed May 30, 2024 (in Japanese). https://www.mhlw.go.jp/content/10900000/000987057.pdf."
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-024-01010-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}