SARS-CoV-2 Clearance from Andrographis paniculata, Boesenbergia rotunda, and Favipiravir among Mild COVID-19 Cases in Klong Prem Central Prison during Mid-2021: a Retrospective Study
et al., OSIR, 15:4, Dec 2022
Retrospective 120 patients in Thailand, showing improved viral clearance with andrographis compared with favipiravir.
|
risk of no viral clearance, 18.8% lower, RR 0.81, p = 0.61, treatment 13 of 30 (43.3%), control 16 of 30 (53.3%), NNT 10, day 14.
|
|
time to viral-, 30.8% lower, relative time 0.69, treatment 30, control 30, andrographis vs. favipiravir.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Prempree et al., 31 Dec 2022, retrospective, Thailand, peer-reviewed, 9 authors, this trial compares with another treatment - results may be better when compared to placebo.
SARS-CoV-2 Clearance from Andrographis paniculata, Boesenbergia rotunda, and Favipiravir among Mild COVID-19 Cases in Klong Prem Central Prison during Mid-2021: a Retrospective Study
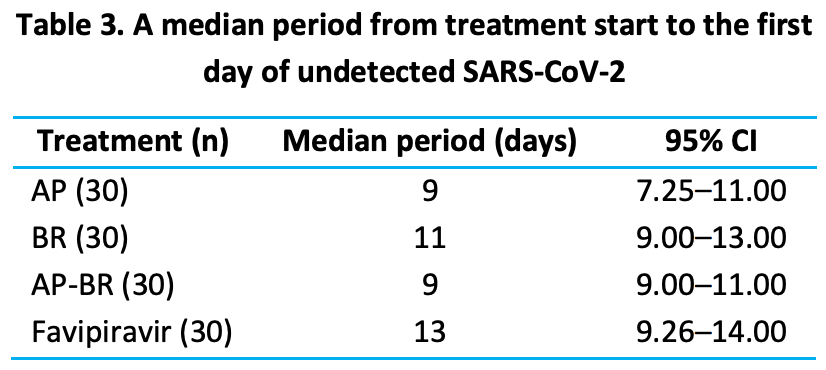
This study aims to assess the effectiveness of two herbal medicines, Andrographis paniculata (Burm.f.) Nees capsule (AP) and Boesenbergia rotunda (Linn.) Mansf. extract capsule (BR), on the rate of SARS-CoV-2 virus clearance among inmates of Klong Prem Central Prison, Bangkok. Cases with mild COVID-19 were allocated into four groups: four capsules of AP thrice daily (n=30), one capsule of BR once daily (n=30), a combination of AP and BR (AP-BR) (n=30), or favipiravir (n=30) for five days. The primary outcome was time until undetected SARS-CoV-2 infection after starting treatment. The median period of SARS-CoV-2 clearance was shorter in the AP and AP-BR groups (9 days) compared to the BR (11 days) and favipiravir (13 days) groups. No one developed pneumonia; however, one participant in the AP group developed hyperkalemia. Our results suggest that A. paniculata with or without B. rotunda may be used as an alternative treatment for mild COVID-19 when access to favipiravir is limited. Further clinical trials are needed to determine their efficacy and safety.
Authors' Contributions PP guided and revised the manuscript. AM and PK revised the manuscript. MT analyzed study data and wrote the first draft of the manuscript. TP analyzed study data. KC, CC, DM, and TK carried out the research. All authors approved the final manuscript.
References
Bethesda, Md), ClinicalTrials.gov; 2021 [cited
Hu, Wu, Logue, Blondel, Lai et al., Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: a systematic review and meta-analysis
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.10.069
Kanjanasirirat, Suksatu, Manopwisedjaroen, Munyoo, Tuchinda et al., High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci Rep, doi:10.1038/s41598-020-77003-3
Numthavaj, Andrographis paniculata vs Boesenbergia rotunda vs control in asymptomatic COVID-19
Rattanaumpawan, Jirajariyavej, Lerdlamyong, Palavutitotai, Saiyarin, Real-world effectiveness and optimal dosage of favipiravir for treatment of COVID-19: results from a multicenter observational study in Thailand, Antibiotics (Basel), doi:10.3390/antibiotics11060805
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Shu, He, Sun, Lin, Lu et al., Factors influencing viral clearance in mild COVID-19 and clinical characteristics of asymptomatic participants, Biomed Res Int, doi:10.1155/2021/5909612
Tanwettiyanont, Piriyachananusorn, Sangsoi, Boonsong, Sunpapoa et al., Use of Andrographis paniculata (Burm.f.) Wall. ex Nees and risk of pneumonia in hospitalised participants with mild coronavirus disease 2019: a retrospective cohort study, Front Med (Lausanne), doi:10.3389/fmed.2022.947373
Ucan, Cerci, Efe, Akgun, Ozmen et al., Benefits of treatment with favipiravir in hospitalized participants for COVID-19: a retrospective observational casecontrol study, Virol J, doi:10.1186/s12985-021-01577-1
Wanaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Efficacy and safety of Andrographis paniculata extract in participants with mild COVID-19: a randomized controlled trial, Arch Intern Med Res, doi:10.26502/aimr.0125
Zhang, Lv, Zhou, Xie, Xu et al., Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, openlabel and randomized controlled trial, Phytother Res, doi:10.1002/ptr.7141