Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand
et al., Antibiotics, doi:10.3390/antibiotics11060805, Jun 2022
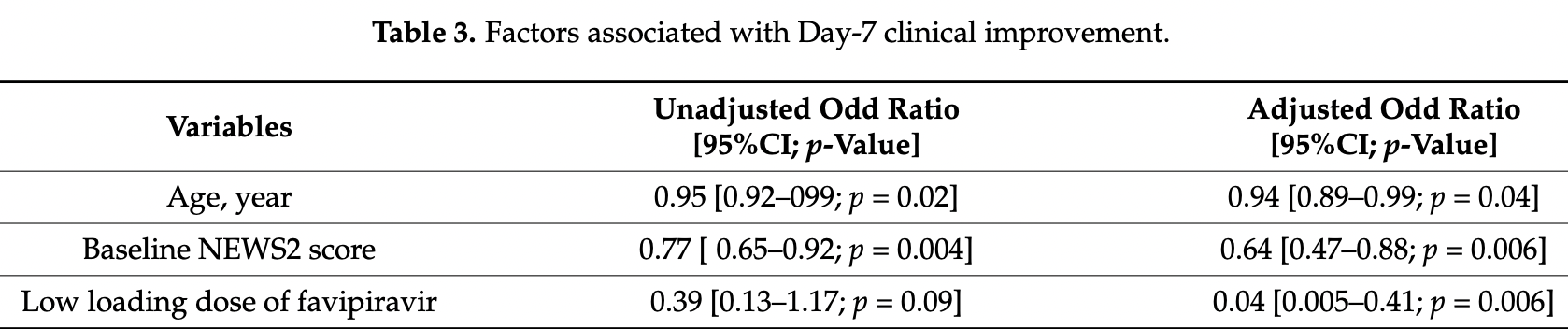
Retrospective 63 hospitalized patients in Thailand treated with favipiravir showing a lower favipiravir loading dose negatively associated with clinical improvement.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Rattanaumpawan et al., 15 Jun 2022, retrospective, Thailand, peer-reviewed, 5 authors.
Contact: pinyo.rat@mahidol.ac.th (corresponding author), jsupunee@yahoo.com, ornmd@live.com, nattawan.pala@gmail.com, ooyaoi@hotmail.com.
Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand
Antibiotics, doi:10.3390/antibiotics11060805
Favipiravir is a broad-spectrum oral antiviral agent that shows in vitro activity against SARS-CoV-2. Presently, data on the real-world effectiveness and optimal dosage of favipiravir for treating COVID-19 are limited. We conducted a retrospective observational study of hospitalized adult patients with COVID-19 at five tertiary care hospitals in Thailand. We reviewed patient charts to obtain all necessary data. Among 247 COVID-19 patients, 63 (23.0%) received ≥1 dose of favipiravir. Of these 63 patients, 61.9% were male with a median age of 48 years (range 22-85 years), 27.0% required an O 2 nasal cannula, 9.5% required non-invasive ventilation and/or high-flow O 2 therapy, and 6.4% required invasive mechanical ventilation and/or ECMO. The median baseline NEWS2 score was 5 (0-16). The Day-7 clinical improvement rate [95%CI] was 66.7% [53.7-78.0%] in all patients, 92.5% [75.7-99.1%] in patients who did not require O 2 supplementation, and 47.2% [0.4-64.5%] in patients who required O 2 supplementation. No life-threatening adverse events were identified. The 28-day mortality rate was 4.8%. A multivariate analysis revealed three poor prognostic factors for Day-7 clinical improvement (odds ratio (95%CI); p-value): older age (0.94 (0.89-0.99); p = 0.04), a higher baseline NEWS2 score (0.64 (0.47-0.88); p = 0.006), and a lower favipiravir loading dose (≤45 mg/kg/day) (0.04 (0.005-0.4); p = 0.006). In conclusion, our study reports the promising effectiveness of favipiravir for treating COVID-19 patients. In addition to older age and a high baseline NEWS2 score, a low loading dose of favipiravir (≤45 mg/kg/day) was also identified as a poor prognostic factor for early clinical improvement. Further studies to explore the optimal dose and the optimal timing of drug initiation for favipiravir should be performed.
Author Contributions: Conceptualization, P.R.; methodology, P.R., investigation, S.J., K.L., N.P. and J.S.; writing-original draft preparation, P.R., S.J., K.L., N.P. and J.S.; writing-review and editing, P.R., funding acquisition, P.R. All authors have read and agreed to the published version of the manuscript. Funding: This research was funded by Siriraj Research Fund, Grant number (IO) R016333038, Faculty of Medicine Siriraj Hospital, Mahidol University. The APC was funded by Faculty of Medicine Siriraj Hospital, Mahidol University.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of all involved hospitals. The Siriraj IRB approved the protocol on 1 May 2020 (certification of approval number: Si 357/2020). Informed Consent Statement: Written inform consent was waived due to a retrospective study design.
Conflicts of Interest: The authors declare no conflict of interest.
References
Bai, Mu, Kargbo, Song, Niu et al., Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, Clin. Infect. Dis, doi:10.1093/cid/ciw571
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Preliminary Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Bosaeed, Alharbi, Mahmoud, Alrehily, Bahlaq et al., Efficacy of favipiravir in adults with mild COVID-19: A randomized, double-blind, multicentre, placebocontrolled clinical trial, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.12.026
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering, doi:10.1016/j.eng.2020.03.007
Chen, Huang, Cheng, Wu, Chen et al., Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial, doi:10.1101/2020.03.17.20037432
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial, Front. Pharmacol, doi:10.3389/fphar.2021.683296
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial, Clin. Infect. Dis, doi:10.1093/cid/ciab962
Dabbous, El-Sayed, El Assal, Elghazaly, Ebeid et al., Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial, Sci. Rep, doi:10.1038/s41598-021-98683-5
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob. Agents Chemother, doi:10.1128/AAC.01897-20
Fischer, Eron, Jr, Holman, Cohen et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci. Transl. Med, doi:10.1126/scitranslmed.abl7430
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc. Jpn. Acad. Ser. B, doi:10.2183/pjab.93.027
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Goyal, Choi, Pinheiro, Schenck, Chen et al., Clinical Characteristics of Covid-19 in New York City, N. Engl. J. Med, doi:10.1056/NEJMc2010419
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Hung, Ghula, Aziz, Makram, Tawfik et al., The efficacy and adverse effects of favipiravir on patients with COVID-19: A systematic review and meta-analysis of published clinical trials and observational studies, Int. J. Infect. Dis
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ, doi:10.1136/bmj.n2713
Manaf, Nitya, Dhuha, Abdulkarim, Fatema et al., Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease, Sci. Rep, doi:10.1038/s41598-022-08794-w
Nguyen, Guedj, Anglaret, Laouenan, Madelain et al., Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted, PLOS Negl. Trop. Dis, doi:10.1371/journal.pntd.0005389
Pavan, Saiprasad, Akash, Akash, Aneesh et al., Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting, Int. J. Gen. Med, doi:10.2147/IJGM.S349241
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.11.142
Wang, Fan, Salam, Horby, Hayden et al., Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection, J. Infect. Dis, doi:10.1093/infdis/jiz656
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Zhao, Zhang, Zhu, Chen, Chen et al., Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, open-label, randomized trial, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.107702
DOI record:
{
"DOI": "10.3390/antibiotics11060805",
"ISSN": [
"2079-6382"
],
"URL": "http://dx.doi.org/10.3390/antibiotics11060805",
"abstract": "<jats:p>Favipiravir is a broad-spectrum oral antiviral agent that shows in vitro activity against SARS-CoV-2. Presently, data on the real-world effectiveness and optimal dosage of favipiravir for treating COVID-19 are limited. We conducted a retrospective observational study of hospitalized adult patients with COVID-19 at five tertiary care hospitals in Thailand. We reviewed patient charts to obtain all necessary data. Among 247 COVID-19 patients, 63 (23.0%) received ≥1 dose of favipiravir. Of these 63 patients, 61.9% were male with a median age of 48 years (range 22–85 years), 27.0% required an O2 nasal cannula, 9.5% required non-invasive ventilation and/or high-flow O2 therapy, and 6.4% required invasive mechanical ventilation and/or ECMO. The median baseline NEWS2 score was 5 (0–16). The Day-7 clinical improvement rate [95%CI] was 66.7% [53.7–78.0%] in all patients, 92.5% [75.7–99.1%] in patients who did not require O2 supplementation, and 47.2% [0.4–64.5%] in patients who required O2 supplementation. No life-threatening adverse events were identified. The 28-day mortality rate was 4.8%. A multivariate analysis revealed three poor prognostic factors for Day-7 clinical improvement (odds ratio (95%CI); p-value): older age (0.94 (0.89–0.99); p = 0.04), a higher baseline NEWS2 score (0.64 (0.47–0.88); p = 0.006), and a lower favipiravir loading dose (≤45 mg/kg/day) (0.04 (0.005–0.4); p = 0.006). In conclusion, our study reports the promising effectiveness of favipiravir for treating COVID-19 patients. In addition to older age and a high baseline NEWS2 score, a low loading dose of favipiravir (≤45 mg/kg/day) was also identified as a poor prognostic factor for early clinical improvement. Further studies to explore the optimal dose and the optimal timing of drug initiation for favipiravir should be performed.</jats:p>",
"alternative-id": [
"antibiotics11060805"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2964-6732",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rattanaumpawan",
"given": "Pinyo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jirajariyavej",
"given": "Supunnee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lerdlamyong",
"given": "Kanokorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palavutitotai",
"given": "Nattawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saiyarin",
"given": "Jatuporn",
"sequence": "additional"
}
],
"container-title": "Antibiotics",
"container-title-short": "Antibiotics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
16
]
],
"date-time": "2022-06-16T02:17:01Z",
"timestamp": 1655345821000
},
"deposited": {
"date-parts": [
[
2022,
6,
16
]
],
"date-time": "2022-06-16T02:28:14Z",
"timestamp": 1655346494000
},
"funder": [
{
"award": [
"R016333038"
],
"name": "Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand"
}
],
"indexed": {
"date-parts": [
[
2022,
6,
16
]
],
"date-time": "2022-06-16T03:13:08Z",
"timestamp": 1655349188417
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2022,
6,
15
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2022,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
15
]
],
"date-time": "2022-06-15T00:00:00Z",
"timestamp": 1655251200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2079-6382/11/6/805/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "805",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
6,
15
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard\nhttps://covid19.who.int/"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1136/bmj.n2713",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1093/cid/ciw571",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.3389/fphar.2021.683296",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1093/cid/ciab962",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1038/s41598-021-98683-5",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.intimp.2021.107702",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1038/s41598-022-08794-w",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.2147/IJGM.S349241",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.ijid.2022.04.035",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/infdis/jiz656",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1371/journal.pntd.0005389",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1056/NEJMc2010419",
"doi-asserted-by": "publisher",
"key": "ref25"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2079-6382/11/6/805"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Pharmacology, Toxicology and Pharmaceutics",
"Biochemistry",
"Microbiology"
],
"subtitle": [],
"title": "Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand",
"type": "journal-article",
"volume": "11"
}