Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2021.683296, Sep 2021
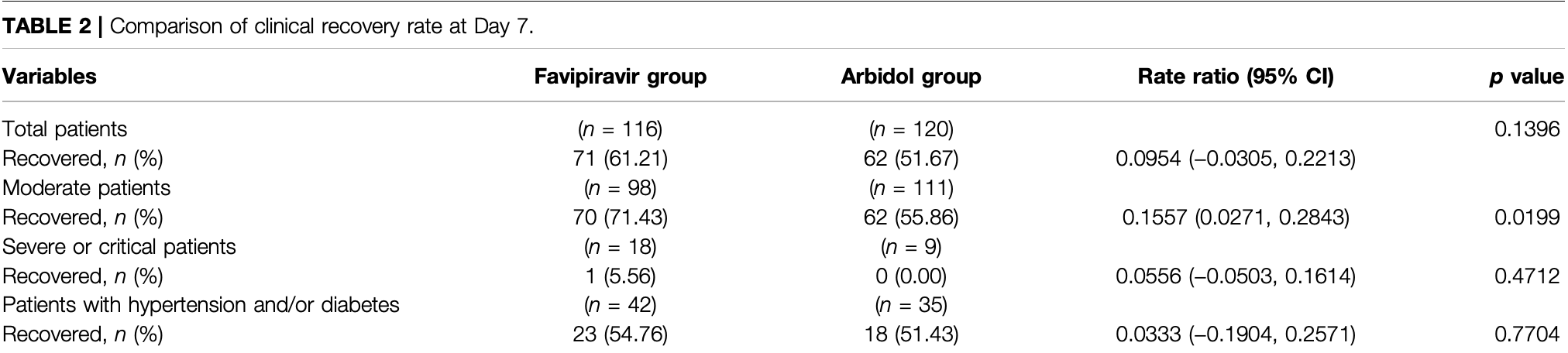
Very late stage (9 days from symptom onset) RCT with 116 favipiravir patients and 120 arbidol patients in China, showing no significant difference in clinical recovery (relief of fever and cough, respiratory frequency ≤24 times/min, and oxygen saturation ≥98%), however the time to resolution of fever and cough was significantly lower with favipiravir. ChiCTR2000030254.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments15.
|
risk of ICU admission, 3.4% higher, RR 1.03, p = 1.00, treatment 2 of 116 (1.7%), control 2 of 120 (1.7%).
|
|
risk of respiratory failure, 74.1% lower, RR 0.26, p = 0.37, treatment 1 of 116 (0.9%), control 4 of 120 (3.3%), NNT 40.
|
|
risk of oxygen therapy, 19.5% lower, RR 0.80, p = 0.42, treatment 21 of 116 (18.1%), control 27 of 120 (22.5%), NNT 23.
|
|
risk of progression to dyspnea, 70.4% lower, RR 0.30, p = 0.03, treatment 4 of 116 (3.4%), control 14 of 120 (11.7%), NNT 12.
|
|
risk of dyspnea, 10.3% lower, RR 0.90, p = 0.84, treatment 13 of 116 (11.2%), control 15 of 120 (12.5%), NNT 77.
|
|
risk of no recovery, 19.7% lower, RR 0.80, p = 0.15, treatment 45 of 116 (38.8%), control 58 of 120 (48.3%), NNT 10, day 7, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Chen et al., 2 Sep 2021, Randomized Controlled Trial, China, peer-reviewed, 14 authors, average treatment delay 9.0 days, this trial compares with another treatment - results may be better when compared to placebo.
Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial
Frontiers in Pharmacology, doi:10.3389/fphar.2021.683296
Background: In addition to supportive therapy, antiviral therapy is an effective treatment for coronavirus disease 2019 . Objective: To compare the efficacy and safety of favipiravir and umifenovir (Arbidol) to treat COVID-19 patients. Methods: We conducted a prospective, randomized, controlled, open-label multicenter trial involving adult patients with COVID-19. Enrolled patients with initial symptoms within 12 days were randomly assigned in a 1:1 ratio to receive conventional therapy plus Arbidol (200 mg*3/day) or favipiravir (1600 mg*2/first day followed by 600 mg*2/day) for 7 days. The primary outcome was the clinical recovery rate at day 7 of drug administration (relief for pyrexia and cough, respiratory frequency ≤24 times/min; oxygen saturation ≥98%). Latency to relief for pyrexia and cough and the rate of auxiliary oxygen therapy (AOT) or noninvasive mechanical ventilation (NMV)/mechanical ventilation (MV) were the secondary outcomes. Safety data were collected for 17 days. Results: A total of 240 enrolled COVID-19 patients underwent randomization; 120 patients were assigned to receive favipiravir (116 assessed), and 120 patients were assigned to receive Arbidol (120 assessed). The clinical recovery rate at day 7 of drug administration did not significantly differ between the favipiravir group (71/116) and Arbidol group (62/120) (p 0.1396, difference in recovery rate: 0.0954; 95% CI: −0.0305∼0.2213). Favipiravir contributed to relief for both pyrexia (difference: 1.70 days, p < 0.0001) and cough (difference: 1.75 days, p < 0.0001). No difference was observed in the
ETHICS STATEMENT The studies involving human participants were reviewed and approved by The study was approved by the Institutional Ethics Committee (No. 2020040). The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS CC, YiZ, JH, and PY contributed equally to this paper. CC, JH, JW, and XW conceived and designed the study. CC, YiZ, JH, PY, ZC, JW, and YoZ contributed to patient recruitment, data collection, data analysis and data interpretation. CC, YiZ, JH, PY, SC, BC, ML, YL, and LJ wrote the first draft of the manuscript. CC, JH, and XW provided administrative, technical, or material support. JH and XW supervised the study. CC, YiZ, JH, JW, YoZ, BC, JZ, and XW contributed to the critical revision of the manuscript for important intellectual content. All authors reviewed and approved the final version of the manuscript.
FUNDING This work was supported by the National Key Research and Development Program of China (2020YFC0844400). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.683296/ full#supplementary-material Conflict of Interest: Author YZ is employed by the company Euler..
References
Dong, Hu, Gao, Discovering Drugs to Treat Coronavirus Disease 2019 (COVID-19), Drug Discov. Ther, doi:10.5582/ddt.2020.01012
Du, Chen, Favipiravir: Pharmacokinetics and Concerns about Clinical Trials for 2019-nCoV Infection, Clin. Pharmacol. Ther, doi:10.1002/cpt.1844
Furuta, Takahashi, Fukuda, Kuno, Kamiyama et al., In Vitro and In Vivo Activities of Anti-influenza Virus Compound T-705, Antimicrob. Agents Chemother, doi:10.1128/aac.46.4.977-981.2002
Goldhill, Te, Velthuis, Fletcher, Langat et al., The Mechanism of Resistance to Favipiravir in Influenza, Proc. Natl. Acad. Sci. U S A, doi:10.1073/pnas.1811345115
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Hulseberg, Fénéant, Szymańska-De Wijs, Kessler, Nelson et al., Arbidol and Other Low-Molecular-Weight Drugs that Inhibit Lassa and Ebola Viruses, J. Virol, doi:10.1128/JVI.02185-18
Leneva, Burtseva, Yatsyshina, Fedyakina, Kirillova et al., Virus Susceptibility and Clinical Effectiveness Frontiers in Pharmacology | www.frontiersin
Leneva, Falynskova, Makhmudova, Poromov, Yatsyshina et al., Umifenovir Susceptibility Monitoring and Characterization of Influenza Viruses Isolated during ARBITR Clinical Study, J. Med. Virol, doi:10.1002/jmv.25358
Li, Guan, Wu, Wang, Zhou et al., Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2001316
Mentre, Taburet, Guedj, Anglaret, Keita et al., Dose Regimen of Favipiravir for Ebola Virus Disease, Lancet Infect. Dis, doi:10.1016/S1473-3099(14)71047-3
Nojomi, Yassin, Keyvani, Makiani, Roham et al., Effect of Arbidol (Umifenovir) on COVID-19: a Randomized Controlled Trial, BMC Infect. Dis, doi:10.26442/00403660.2019.03.000127
Roser, Ritchie, Ortiz-Ospina, Coronavirus Disease (COVID-19)-Statistics and Research
Shi, Xiong, He, Deng, Li et al., Antiviral Activity of Arbidol against Influenza A Virus, Respiratory Syncytial Virus, Rhinovirus, coxsackie Virus and Adenovirus In Vitro and In Vivo, Arch. Virol, doi:10.1007/s00705-007-0974-5
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Who, Coronavirus Disease
Xu, Chen, Yuan, Yi, Ding et al., Clinical Efficacy of Arbidol in Patients with 2019 Novel Coronavirus-Infected Pneumonia: A Retrospective Cohort Study, doi:https://ssrn.com/abstract=3542148%20or%20doi:%2010.2139/ssrn.3542148
Zhang, Wang, Peng, Peng, Zhang et al., Potential of Arbidol for post-exposure Prophylaxis of COVID-19 Transmission: a Preliminary Report of a Retrospective Cohort Study, Curr. Med. Sci, doi:10.1007/s11596-020-2203-3
DOI record:
{
"DOI": "10.3389/fphar.2021.683296",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2021.683296",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> In addition to supportive therapy, antiviral therapy is an effective treatment for coronavirus disease 2019 (COVID-19).</jats:p><jats:p><jats:bold>Objective:</jats:bold> To compare the efficacy and safety of favipiravir and umifenovir (Arbidol) to treat COVID-19 patients.</jats:p><jats:p><jats:bold>Methods:</jats:bold> We conducted a prospective, randomized, controlled, open-label multicenter trial involving adult patients with COVID-19. Enrolled patients with initial symptoms within 12 days were randomly assigned in a 1:1 ratio to receive conventional therapy plus Arbidol (200 mg*3/day) or favipiravir (1600 mg*2/first day followed by 600 mg*2/day) for 7 days. The primary outcome was the clinical recovery rate at day 7 of drug administration (relief for pyrexia and cough, respiratory frequency ≤24 times/min; oxygen saturation ≥98%). Latency to relief for pyrexia and cough and the rate of auxiliary oxygen therapy (AOT) or noninvasive mechanical ventilation (NMV)/mechanical ventilation (MV) were the secondary outcomes. Safety data were collected for 17 days.</jats:p><jats:p><jats:bold>Results:</jats:bold> A total of 240 enrolled COVID-19 patients underwent randomization; 120 patients were assigned to receive favipiravir (116 assessed), and 120 patients were assigned to receive Arbidol (120 assessed). The clinical recovery rate at day 7 of drug administration did not significantly differ between the favipiravir group (71/116) and Arbidol group (62/120) (<jats:italic>p</jats:italic> = 0.1396, difference in recovery rate: 0.0954; 95% CI: −0.0305∼0.2213). Favipiravir contributed to relief for both pyrexia (difference: 1.70 days, <jats:italic>p</jats:italic> &lt; 0.0001) and cough (difference: 1.75 days, <jats:italic>p</jats:italic> &lt; 0.0001). No difference was observed in the AOT or NMV/MV rate (both <jats:italic>p</jats:italic> &gt; 0.05). The most frequently observed favipiravir-associated adverse event was increased serum uric acid (16/116, OR: 5.52, <jats:italic>p</jats:italic> = 0.0014).</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> Among patients with COVID-19, favipiravir, compared to Arbidol, did not significantly improve the clinical recovery rate at day 7. Favipiravir significantly improved the latency to relieve pyrexia and cough. Adverse effects caused by favipiravir are mild and manageable.</jats:p>",
"alternative-id": [
"10.3389/fphar.2021.683296"
],
"author": [
{
"affiliation": [],
"family": "Chen",
"given": "Chang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Jianying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yin",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Zhenshun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Jianyuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Song",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yongxi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Mengxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Yongwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ju",
"given": "Lingao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Jingyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xinghuan",
"sequence": "additional"
}
],
"container-title": [
"Frontiers in Pharmacology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
9,
4
]
],
"date-time": "2021-09-04T02:33:45Z",
"timestamp": 1630722825000
},
"deposited": {
"date-parts": [
[
2021,
9,
4
]
],
"date-time": "2021-09-04T02:33:50Z",
"timestamp": 1630722830000
},
"funder": [
{
"DOI": "10.13039/501100012166",
"award": [
"2020YFC0844400"
],
"doi-asserted-by": "publisher",
"name": "National Key Research and Development Program of China"
}
],
"indexed": {
"date-parts": [
[
2021,
11,
12
]
],
"date-time": "2021-11-12T07:06:53Z",
"timestamp": 1636700813288
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "electronic",
"value": "1663-9812"
}
],
"issued": {
"date-parts": [
[
2021,
9,
2
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
2
]
],
"date-time": "2021-09-02T00:00:00Z",
"timestamp": 1630540800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2021.683296/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
9,
2
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
2
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.5582/ddt.2020.01012",
"article-title": "Discovering Drugs to Treat Coronavirus Disease 2019 (COVID-19)",
"author": "Dong",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "Drug Discov. Ther.",
"key": "B1",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: Pharmacokinetics and Concerns about Clinical Trials for 2019-nCoV Infection",
"author": "Du",
"doi-asserted-by": "publisher",
"first-page": "242",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "B2",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1128/aac.46.4.977-981.2002",
"article-title": "In Vitro and In Vivo Activities of Anti-influenza Virus Compound T-705",
"author": "Furuta",
"doi-asserted-by": "publisher",
"first-page": "977",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "B3",
"volume": "46",
"year": "2002"
},
{
"DOI": "10.1073/pnas.1811345115",
"article-title": "The Mechanism of Resistance to Favipiravir in Influenza",
"author": "Goldhill",
"doi-asserted-by": "publisher",
"first-page": "11613",
"journal-title": "Proc. Natl. Acad. Sci. U S A.",
"key": "B4",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "B5",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1128/JVI.02185-18",
"article-title": "Arbidol and Other Low-Molecular-Weight Drugs that Inhibit Lassa and Ebola Viruses",
"author": "Hulseberg",
"doi-asserted-by": "publisher",
"journal-title": "J. Virol.",
"key": "B6",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1016/j.ijid.2016.01.001",
"article-title": "Virus Susceptibility and Clinical Effectiveness of Anti-influenza Drugs during the 2010-2011 Influenza Season in Russia",
"author": "Leneva",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Int. J. Infect. Dis.",
"key": "B7",
"volume": "43",
"year": "2016"
},
{
"DOI": "10.1002/jmv.25358",
"article-title": "Umifenovir Susceptibility Monitoring and Characterization of Influenza Viruses Isolated during ARBITR Clinical Study",
"author": "Leneva",
"doi-asserted-by": "publisher",
"first-page": "588",
"journal-title": "J. Med. Virol.",
"key": "B8",
"volume": "91",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2001316",
"article-title": "Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "1199",
"journal-title": "N. Engl. J. Med.",
"key": "B9",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(14)71047-3",
"article-title": "Dose Regimen of Favipiravir for Ebola Virus Disease",
"author": "Mentre",
"doi-asserted-by": "publisher",
"first-page": "150",
"journal-title": "Lancet Infect. Dis.",
"key": "B10",
"volume": "15",
"year": "2015"
},
{
"key": "B11",
"volume-title": "Chinese Diagnosis and Treatment Plan of COVID-19 Patients",
"year": ""
},
{
"key": "B12",
"volume-title": "Chinese Diagnosis and Treatment Plan of COVID-19 Patients",
"year": ""
},
{
"DOI": "10.1186/s12879-020-05698-w",
"article-title": "Effect of Arbidol (Umifenovir) on COVID-19: a Randomized Controlled Trial",
"author": "Nojomi",
"doi-asserted-by": "publisher",
"first-page": "954",
"journal-title": "BMC Infect. Dis.",
"key": "B13",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.26442/00403660.2019.03.000127",
"article-title": "Clinical Efficacy of Umifenovir in Influenza and ARVI (Study ARBITR)",
"author": "Pshenichnaya",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Ter Arkh",
"key": "B14",
"volume": "91",
"year": "2019"
},
{
"key": "B15",
"unstructured": "Coronavirus Disease (COVID-19)-Statistics and Research\n RoserM.\n RitchieH.\n Ortiz-OspinaE.\n 2020"
},
{
"DOI": "10.1007/s00705-007-0974-5",
"article-title": "Antiviral Activity of Arbidol against Influenza A Virus, Respiratory Syncytial Virus, Rhinovirus, coxsackie Virus and Adenovirus In Vitro and In Vivo",
"author": "Shi",
"doi-asserted-by": "publisher",
"first-page": "1447",
"journal-title": "Arch. Virol.",
"key": "B16",
"volume": "152",
"year": "2007"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "JAMA",
"key": "B17",
"volume": "323",
"year": "2020"
},
{
"key": "B18",
"unstructured": "Coronavirus Disease 2019 (COVID-19) Situation Report2020"
},
{
"article-title": "Clinical Efficacy of Arbidol in Patients with 2019 Novel Coronavirus-Infected Pneumonia: A Retrospective Cohort Study",
"author": "Xu",
"key": "B19",
"volume-title": "Lancet",
"year": "2019"
},
{
"DOI": "10.1007/s11596-020-2203-3",
"article-title": "Potential of Arbidol for post-exposure Prophylaxis of COVID-19 Transmission: a Preliminary Report of a Retrospective Cohort Study",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "480",
"journal-title": "Curr. Med. Sci.",
"key": "B20",
"volume": "40",
"year": "2020"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"score": 1,
"short-container-title": [
"Front. Pharmacol."
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": [
"Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "12"
}