Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting
et al., International Journal of General Medicine, doi:10.2147/IJGM.S349241, CTRI/2020/11/029263, May 2022
Prospective single-arm study of 1,083 patients receiving favipiravir in India, showing one death and no new safety issues.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Reddy et al., 3 May 2022, prospective, India, peer-reviewed, 19 authors, study period December 2020 - June 2021, trial CTRI/2020/11/029263.
Contact: sagar.bhagat@glenmarkpharma.com.
Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting
International Journal of General Medicine, doi:10.2147/ijgm.s349241
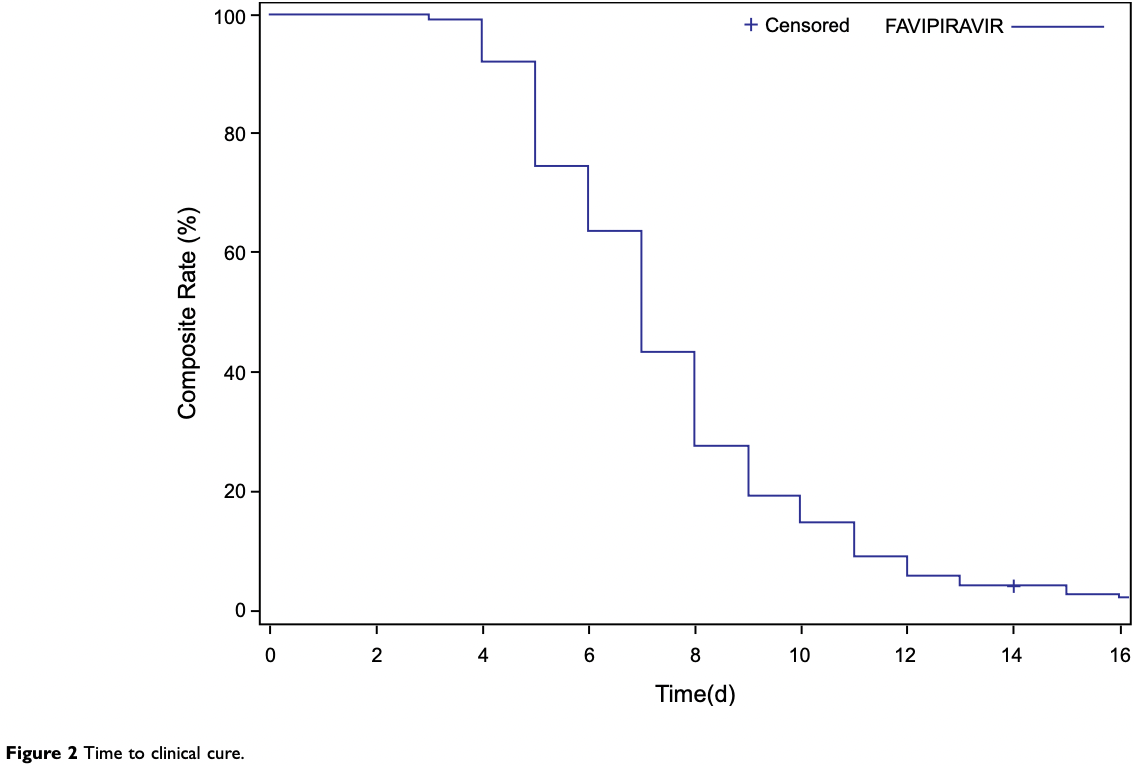
To evaluate the safety and efficacy of favipiravir, which is prescribed for the treatment of patients with mild-to-moderate coronavirus disease 2019 (COVID-19) in India. Patients and Methods: This was a prospective, open-label, multicenter, single-arm postmarketing study conducted in India. Patients with mild-to-moderate COVID-19 received favipiravir (3600 mg [1800 mg orally twice daily] on the first day, followed by 800 mg orally twice daily, up to a maximum of 14 days) as a part of their treatment. The primary endpoints were to evaluate the safety of favipiravir by assessing the number of adverse events (AEs) and treatment-related AEs. The secondary endpoints were to evaluate the efficacy of favipiravir by assessing time to clinical cure, rate of clinical cure, time to pyrexia resolution, rate of oxygen requirement, and all-cause mortality. Results: A total of 1083 patients were enrolled in this study from December 2020 to June 2021. Adverse events were reported in 129 patients (11.9%), 116 (10.7%) of whom had mild AEs. Dose modification or withdrawal of favipiravir treatment was reported in four patients (0.37%). The median time to clinical cure and pyrexia resolution was 7 and 4 days, respectively. A total of 1036 patients (95.8%) exhibited clinical cure by day 14. Oxygen support was required by 15 patients (1.4%). One death was reported, which was unrelated to favipiravir.
Conclusion: In the real-world setting, favipiravir was well-tolerated, and no new safety signals were detected.
Time to Resolution of Pyrexia The median time to resolution of pyrexia in the present study was 4 days. The Kaplan-Meier survival curve for time to resolution of pyrexia in the mITT population is presented in Figure 4 . Additional details on subgroups are provided in the Supplementary Results.
Author Contributions All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure SP, SB, PD, AP, WW, SR, HB are full time employee of Glenmark Pharmaceuticals Ltd. PKR reports grants from Glenmark, during the conduct of the study. MK reports personal fees from Glenmark limited, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Chang, Park, Kim, Park, Risk factors for disease progression in COVID-19 patients, BMC Infect Dis, doi:10.1186/s12879-020-05144-x
Chen, Zhang, Huang, Favipiravir versus arbidol for COVID-19: a randomized clinical trial, medRxiv, doi:10.3389/fphar.2021.683296
Dabbous, Abd-Elsalam, El-Sayed, Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study, Arch Virol, doi:10.1007/s00705-021-04956-9
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res, doi:10.1016/j.antiviral.2013.09.015
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial, Clin Infect Dis, doi:10.1093/cid/ciaa1176
Lou, Liu, Yao, Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial, Eur J Pharm Sci, doi:10.1016/j.ejps.2020.105631
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.3071
Nasir, Murshed, Nazneen, Talha, Survival and biomarkers of COVID-19 patients treated with remdesivir and favipiravir in ICU during the peak of pandemic: a single center study in Bangladesh, J Pharma Res Int
Nasir, Perveen, Saha, Talha, Selina et al., Systematic review on repurposing use of favipiravir against SARS-CoV-2, Journal of General Medicine, doi:10.2147/IJGM.S349241
Panchal, Venugopal, Vora, P5-64: therapeutic effectiveness & safety of favipiravir in COVID-19 patients with risk factors for mortality: clinical practice experience from India, Respirology, doi:10.1111/resp.14150_271
Panchal, Venugopal, Vora, P5-70: effectiveness of favipiravir in COVID-19 patients with multiple (≥2) or less (<2) comorbidities: a real word experience from India, Respirology
Panchal, Venugopal, Vora, P5-93: therapeutic effectiveness and tolerability of favipiravir in mild COVID-19: real world experience from India, Respirology, doi:10.1111/resp.14150_300
Perlman, Another decade, another coronavirus, N Engl J Med, doi:10.1056/NEJMe2001126
Perveen, Nasir, Murshed, Naznin, Ahmed, Remdesivir and favipiravir changes hepato-renal profile in COVID-19 patients: a cross sectional observation in Bangladesh, Journal of General Medicine, doi:10.18535/ijmsci/v8i01.03
Perveen, Nasir, Talha, Selina, Islam, Systematic review on current antiviral therapy in COVID-19 pandemic, Med J Malaysia
Rattanaumpawan, Jirajariyavej, Lerdlamyong, Palavutitotai, Saiyarin, Real-world experience with favipiravir for treatment of COVID-19 in Thailand: results from a multicenter observational study, medRxiv
Turcotte, Meisenberg, Macdonald, Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital, PLoS One, doi:10.1371/journal.pone.0237558
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
DOI record:
{
"DOI": "10.2147/ijgm.s349241",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S349241",
"author": [
{
"affiliation": [],
"family": "Reddy",
"given": "Pavan Kumar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Patil",
"given": "Saiprasad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khobragade",
"given": "Akash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balki",
"given": "Akash",
"sequence": "additional",
"suffix": "Snr"
},
{
"affiliation": [],
"family": "Raj",
"given": "Aneesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalikar",
"given": "Mrunalini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reddy",
"given": "Raghavendra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinde",
"given": "Ravindra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CR",
"given": "Jayanthi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mutha",
"given": "Abhinandan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyilla",
"given": "Nagaraju",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajadhyaksha",
"given": "Girish C",
"sequence": "additional",
"suffix": "Snr"
},
{
"affiliation": [],
"family": "Karnik",
"given": "Niteen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhagat",
"given": "Sagar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pendse",
"given": "Amol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhage",
"given": "Priyanka",
"sequence": "additional",
"suffix": "Snr"
},
{
"affiliation": [],
"family": "Wu",
"given": "Wen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rangwala",
"given": "Shabbir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barkate",
"given": "Hanmant",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
2
]
],
"date-time": "2022-05-02T12:00:30Z",
"timestamp": 1651492830000
},
"deposited": {
"date-parts": [
[
2022,
5,
2
]
],
"date-time": "2022-05-02T12:00:35Z",
"timestamp": 1651492835000
},
"indexed": {
"date-parts": [
[
2022,
5,
2
]
],
"date-time": "2022-05-02T12:41:12Z",
"timestamp": 1651495272822
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
1
]
],
"date-time": "2022-05-01T00:00:00Z",
"timestamp": 1651363200000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=80472",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=80472",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "4551-4563",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2022,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMe2001126",
"author": "Perlman",
"doi-asserted-by": "publisher",
"first-page": "760",
"journal-title": "N Engl J Med",
"key": "ref1",
"volume": "382",
"year": "2020"
},
{
"key": "ref2",
"unstructured": "World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. Accessed October 25, 2021."
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"author": "Furuta",
"doi-asserted-by": "publisher",
"first-page": "446",
"journal-title": "Antiviral Res",
"key": "ref3",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.2183/pjab.93.027",
"author": "Furuta",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "ref4",
"volume": "93",
"year": "2017"
},
{
"key": "ref5",
"unstructured": "WHO R&D Blueprint COVID 19 Experimental Treatments. COVID classification of treatment types; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/covid-classification-of-treatment-types-rev.pdf?sfvrsn=5b90b2f2_1&download=true. Accessed August 16, 2021."
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"author": "Udwadia",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "ref6",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1111/resp.14150_300",
"doi-asserted-by": "crossref",
"key": "ref7",
"unstructured": "Panchal S, Venugopal K, Vora A, et al. P5-93: therapeutic effectiveness and tolerability of favipiravir in mild COVID-19: real world experience from India. Respirology. 2021;26(S3):194. doi:10.1111/resp.14150_300"
},
{
"DOI": "10.1111/resp.14150_271",
"doi-asserted-by": "crossref",
"key": "ref8",
"unstructured": "Panchal S, Venugopal K, Vora A, et al. P5-64: therapeutic effectiveness & safety of favipiravir in COVID-19 patients with risk factors for mortality: clinical practice experience from India. Respirology. 2021;26(S3):182. doi:10.1111/resp.14150_271"
},
{
"key": "ref9",
"unstructured": "Central Drugs Standard Control Organisation, India. List of new drugs approved in the year 2020. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadApprovalNewDrugs/newdrugs%20approval%20july2020.pdf. Accessed August 16, 2021."
},
{
"author": "Nasir",
"first-page": "747",
"journal-title": "Mymensingh Med J",
"key": "ref10",
"volume": "29",
"year": "2020"
},
{
"author": "Perveen",
"first-page": "710",
"journal-title": "Med J Malaysia",
"key": "ref11",
"volume": "75",
"year": "2020"
},
{
"key": "ref12",
"unstructured": "Favipiravir Observational Study Group. Preliminary report of the favipiravir observational study in Japan; 2020. Available from: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200529.pdf. Accessed August 13, 2021."
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"author": "Cai",
"doi-asserted-by": "publisher",
"first-page": "1192",
"journal-title": "Engineering",
"key": "ref13",
"volume": "6",
"year": "2020"
},
{
"author": "Rattanaumpawan",
"journal-title": "medRxiv",
"key": "ref14",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.683296",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref15",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "ref16",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"author": "López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"journal-title": "JAMA",
"key": "ref17",
"volume": "325",
"year": "2021"
},
{
"author": "Nasir",
"first-page": "14",
"journal-title": "J Pharma Res Int",
"key": "ref18",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1111/resp.14150_277",
"doi-asserted-by": "crossref",
"key": "ref19",
"unstructured": "Panchal S, Venugopal K, Vora A, et al. P5-70: effectiveness of favipiravir in COVID-19 patients with multiple (≥2) or less (<2) comorbidities: a real word experience from India. Respirology. 2021;26(S3):184."
},
{
"DOI": "10.1093/cid/ciaa1176",
"author": "Ivashchenko",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "ref20",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1186/s12879-020-05144-x",
"author": "Chang",
"doi-asserted-by": "publisher",
"first-page": "445",
"journal-title": "BMC Infect Dis",
"key": "ref21",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0237558",
"author": "Turcotte",
"doi-asserted-by": "publisher",
"first-page": "e0237558",
"journal-title": "PLoS One",
"key": "ref22",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1007/s00705-021-04956-9",
"author": "Dabbous",
"doi-asserted-by": "publisher",
"first-page": "949",
"journal-title": "Arch Virol",
"key": "ref23",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"author": "Lou",
"doi-asserted-by": "publisher",
"first-page": "105631",
"journal-title": "Eur J Pharm Sci",
"key": "ref24",
"volume": "157",
"year": "2021"
},
{
"DOI": "10.18535/ijmsci/v8i01.03",
"author": "Ara Perveen",
"doi-asserted-by": "publisher",
"first-page": "5196",
"journal-title": "ijmsci",
"key": "ref25",
"volume": "8",
"year": "2021"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/evaluation-of-the-safety-and-efficacy-of-favipiravir-in-adult-indian-p-peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting",
"type": "journal-article",
"volume": "Volume 15"
}