Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial
et al., European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2020.105631, ChiCTR2000029544, Oct 2020
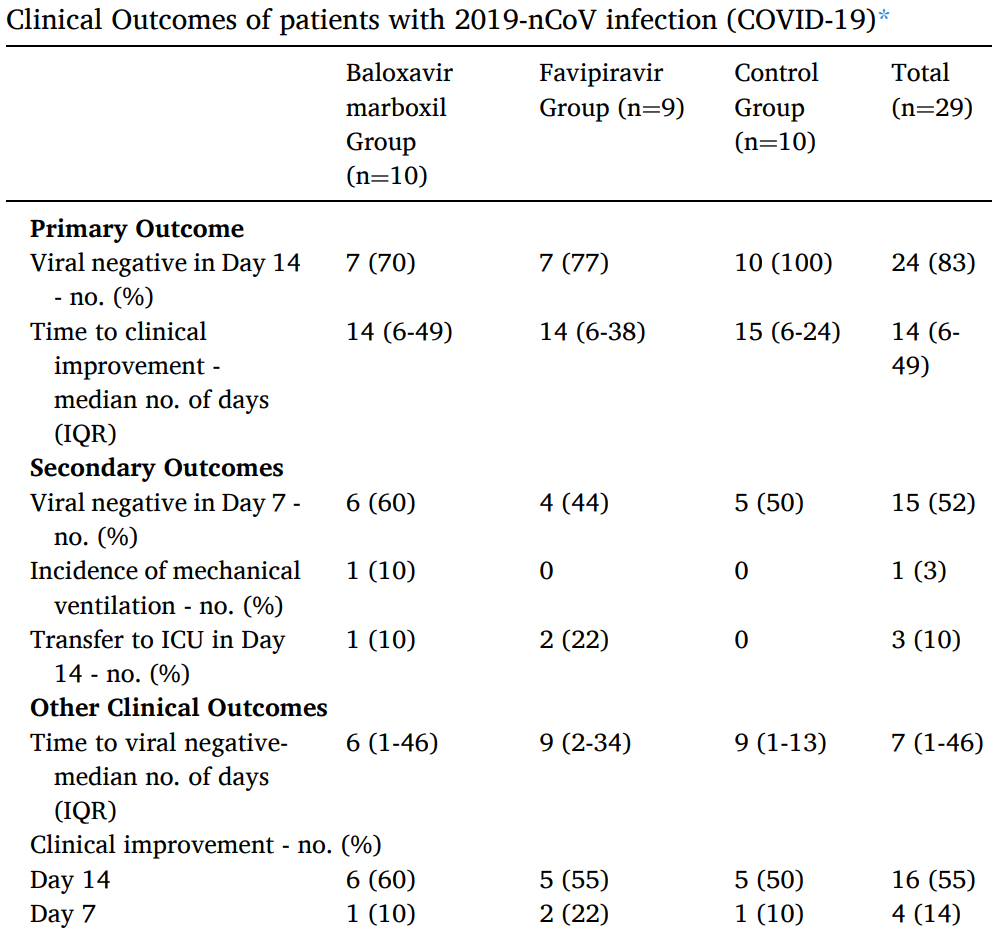
Small late stage RCT with 10 favipiravir, 10 baloxavir marboxil, and 10 control patients in China, showing no significant differences.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments15.
Study covers baloxavir and favipiravir.
|
risk of ICU admission, 422.2% higher, RR 5.22, p = 0.21, treatment 2 of 9 (22.2%), control 0 of 10 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 11.1% lower, RR 0.89, p = 1.00, treatment 4 of 9 (44.4%), control 5 of 10 (50.0%), NNT 18, day 14.
|
|
risk of no recovery, 13.6% lower, RR 0.86, p = 0.58, treatment 7 of 9 (77.8%), control 9 of 10 (90.0%), NNT 8.2, day 7.
|
|
risk of no viral clearance, 422.2% higher, RR 5.22, p = 0.21, treatment 2 of 9 (22.2%), control 0 of 10 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
|
risk of no viral clearance, 11.1% higher, RR 1.11, p = 1.00, treatment 5 of 9 (55.6%), control 5 of 10 (50.0%), day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Lou et al., 25 Oct 2020, Randomized Controlled Trial, China, peer-reviewed, 13 authors, average treatment delay 8.5 days, trial ChiCTR2000029544.
Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial
European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2020.105631
Background: Effective antiviral drugs for COVID-19 are still lacking. This study aims to evaluate the clinical outcomes and plasma concentrations of baloxavir acid and favipiravir in COVID-19 patients. Methods: Favipiravir and baloxavir acid were evaluated for their antiviral activity against SARS-CoV-2 in vitro before the trial initiation. We conducted an exploratory trial with 3 arms involving hospitalized adult patients with COVID-19. Patients were randomized assigned in a 1:1:1 ratio into baloxavir marboxil group, favipiravir group, and control group. The primary outcome was the percentage of subjects with viral negative by Day 14 and the time from randomization to clinical improvement. Virus load reduction, blood drug concentration and clinical presentation were also observed. The trial was registered with Chinese Clinical Trial Registry (ChiCTR 2000029544). Results: Baloxavir acid showed antiviral activity in vitro with the half-maximal effective concentration (EC 50 ) of 5.48 μM comparable to arbidol and lopinavir, but favipiravir didn't demonstrate significant antiviral activity up to 100 μM. Thirty patients were enrolled. The percentage of patients who turned viral negative after 14-day treatment was 70%, 77%, and 100% in the baloxavir marboxil, favipiravir, and control group respectively, with the medians of time from randomization to clinical improvement was 14, 14 and 15 days, respectively. One reason for the lack of virological effect and clinical benefits may be due to insufficient concentrations of these drugs relative to their antiviral activities. One of the limitations of this study is the time from symptom onset to randomization, especially in the baloxavir marboxil and control groups, which is higher than the favipiravir group. Conclusions: Our findings could not prove a benefit of addition of either baloxavir marboxil or favipiravir under the trial dosages to the existing standard treatment.
Supplementary Data: Supplementary materials are available online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author. Adverse events that occurred in more than 1 patient after randomization through day 14 are shown. Some patients had more than one adverse event. There was no death in the trial. ARDS indicates acute respiratory distress syndrome.
CRediT authorship contribution statement
Declaration of Competing Interest We declare no competing interests.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejps.2020.105631.
References
Abdelnabi, Morais, Leyssen, Understanding the Mechanism of the Broad-Spectrum Antiviral Activity of Favipiravir (T-705): Key Role of the F1 Motif of the Viral Polymerase, J Virol, doi:10.1128/jvi.00487-17
Abraham, Morton, Saravolatz, Baloxavir: A Novel Antiviral Agent in the Treatment of Influenza, Clin Infect Dis, doi:10.1093/cid/ciaa107
Bazzoli, Jullien, Le Tiec, Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action, Clin Pharmacokinet, doi:10.2165/11318110-000000000-00000
Cai, Yang, Liu, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, doi:10.1016/j.eng.2020.03.007
Cao, Wang, Wen, A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet, doi:10.1016/S0140-6736(20)30154-9
Chen, Huang, Yin, Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial, doi:medrxiv.10.1101/2020.03.17.20037432
Deng, Zhong, Yu, Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans, Antimicrob Agents Chemother, doi:10.1128/AAC.02282-12
Hayden, Shindo, Influenza virus polymerase inhibitors in clinical development, Curr Opin Infect Dis, doi:10.1097/QCO.0000000000000532
Hayden, Sugaya, Hirotsu, Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents, N Engl J Med, doi:10.1056/NEJMoa1716197
Irie, Nakagawa, Fujita, Pharmacokinetics of Favipiravir in Critically ill Patients with COVID-19, Clin Transl Sci, doi:10.1111/cts.12827
Lou, Liu, Yao, Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial, doi:medrxiv.10.1101/2020.04.29.20085761
Madelain, Oestereich, Graw, Ebola virus dynamics in mice treated with favipiravir, Antiviral Res, doi:10.1016/j.antiviral.2015.08.015
Moltó, Valle, Blanco, Lopinavir/ritonavir pharmacokinetics in HIV and hepatitis C virus co-infected patients without liver function impairment: influence of liver fibrosis, Clin Pharmacokinet, doi:10.2165/00003088-200746010-00005
Nguyen, Guedj, Anglaret, Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0005389
Sun, He, Qiu, Pharmacokinetics of single and multiple oral doses of arbidol in healthy Chinese volunteers, Int J Clin Pharmacol Ther, doi:10.5414/CP201843
Tashima, Crofoot, Tomaka, Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial, AIDS Res Ther, doi:10.1186/1742-6405-11-39
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
DOI record:
{
"DOI": "10.1016/j.ejps.2020.105631",
"ISSN": [
"0928-0987"
],
"URL": "http://dx.doi.org/10.1016/j.ejps.2020.105631",
"alternative-id": [
"S092809872030419X"
],
"article-number": "105631",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "European Journal of Pharmaceutical Sciences"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ejps.2020.105631"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Lou",
"given": "Yan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Liu",
"given": "Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yao",
"given": "Hangping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Xingjiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Su",
"given": "Junwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Kaijin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Rui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Xi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "He",
"given": "Lingjuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Xiaoyang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Qingwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Tingbo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qiu",
"given": "Yunqing",
"sequence": "additional"
}
],
"container-title": [
"European Journal of Pharmaceutical Sciences"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
25
]
],
"date-time": "2020-10-25T14:43:45Z",
"timestamp": 1603637025000
},
"deposited": {
"date-parts": [
[
2021,
6,
18
]
],
"date-time": "2021-06-18T23:24:57Z",
"timestamp": 1624058697000
},
"indexed": {
"date-parts": [
[
2022,
2,
12
]
],
"date-time": "2022-02-12T07:50:23Z",
"timestamp": 1644652223025
},
"is-referenced-by-count": 58,
"issn-type": [
{
"type": "print",
"value": "0928-0987"
}
],
"issued": {
"date-parts": [
[
2021,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T00:00:00Z",
"timestamp": 1612137600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S092809872030419X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S092809872030419X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105631",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
2
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"article-title": "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "514",
"journal-title": "Lancet",
"key": "10.1016/j.ejps.2020.105631_bib0001",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.ejps.2020.105631_bib0002",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1716197",
"article-title": "Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "913",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ejps.2020.105631_bib0003",
"volume": "379",
"year": "2018"
},
{
"DOI": "10.1097/QCO.0000000000000532",
"article-title": "Influenza virus polymerase inhibitors in clinical development",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "176",
"journal-title": "Curr Opin Infect Dis",
"key": "10.1016/j.ejps.2020.105631_bib0004",
"volume": "32",
"year": "2019"
},
{
"DOI": "10.1128/JVI.00487-17",
"article-title": "Understanding the Mechanism of the Broad-Spectrum Antiviral Activity of Favipiravir (T-705): Key Role of the F1 Motif of the Viral Polymerase",
"author": "Abdelnabi",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "J Virol",
"key": "10.1016/j.ejps.2020.105631_bib0005",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.1371/journal.pntd.0005389",
"article-title": "Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "PLoS Negl Trop Dis",
"key": "10.1016/j.ejps.2020.105631_bib0006",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1093/cid/ciaa107",
"article-title": "Baloxavir: A Novel Antiviral Agent in the Treatment of Influenza",
"author": "Abraham",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis.",
"key": "10.1016/j.ejps.2020.105631_bib0007",
"year": "2020"
},
{
"DOI": "10.2165/00003088-200746010-00005",
"article-title": "Lopinavir/ritonavir pharmacokinetics in HIV and hepatitis C virus co-infected patients without liver function impairment: influence of liver fibrosis",
"author": "Moltó",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/j.ejps.2020.105631_bib0008",
"volume": "46",
"year": "2007"
},
{
"DOI": "10.5414/CP201843",
"article-title": "Pharmacokinetics of single and multiple oral doses of arbidol in healthy Chinese volunteers",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "423",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "10.1016/j.ejps.2020.105631_bib0009",
"volume": "51",
"year": "2013"
},
{
"DOI": "10.1128/AAC.02282-12",
"article-title": "Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "1743",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ejps.2020.105631_bib0010",
"volume": "57",
"year": "2013"
},
{
"DOI": "10.1186/1742-6405-11-39",
"article-title": "Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial",
"author": "Tashima",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "AIDS Res Ther",
"key": "10.1016/j.ejps.2020.105631_bib0011",
"volume": "11",
"year": "2014"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med.",
"key": "10.1016/j.ejps.2020.105631_bib0012",
"year": "2020"
},
{
"DOI": "10.2165/11318110-000000000-00000",
"article-title": "Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action",
"author": "Bazzoli",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/j.ejps.2020.105631_bib0013",
"volume": "49",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2015.08.015",
"article-title": "Ebola virus dynamics in mice treated with favipiravir",
"author": "Madelain",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ejps.2020.105631_bib0014",
"volume": "123",
"year": "2015"
},
{
"article-title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"author": "Cai",
"journal-title": "Engineering (Beijing)",
"key": "10.1016/j.ejps.2020.105631_bib0015",
"year": "2020"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2020.105631_bib0016",
"unstructured": "Chen, C., Huang, J.Y., Yin, P., 2020. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medrxiv. 10.1101/2020.03.17.20037432."
},
{
"DOI": "10.1111/cts.12827",
"article-title": "Pharmacokinetics of Favipiravir in Critically ill Patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"journal-title": "Clin Transl Sci.",
"key": "10.1016/j.ejps.2020.105631_bib0017",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.29.20085761",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ejps.2020.105631_bib0018",
"unstructured": "Lou, Y., Liu, L., Yao, H.P., et al. 2020. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. medrxiv. 10.1101/2020.04.29.20085761."
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"score": 1,
"short-container-title": [
"European Journal of Pharmaceutical Sciences"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmaceutical Science"
],
"subtitle": [],
"title": [
"Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "157"
}