Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
et al., Engineering, doi:10.1016/j.eng.2020.03.007, Mar 2020
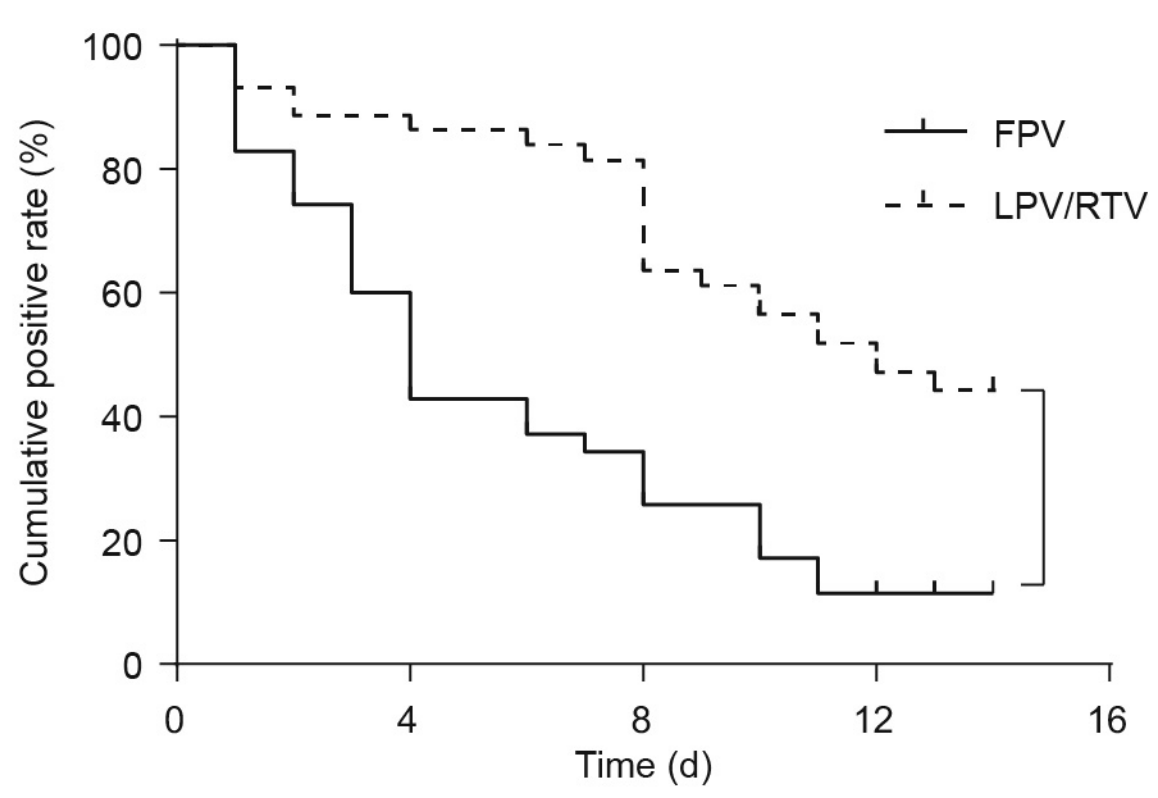
Comparison of 35 FPV patients and 35 LPV/RTV patients, showing significant improvements in chest CT and faster viral clearance with FPV.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments15.
|
risk of no improvement in CT, 68.7% lower, OR 0.31, p = 0.04, treatment 35, control 45, inverted to make OR<1 favor treatment, multivariate, RR approximated with OR.
|
|
risk of no viral clearance, 70.9% lower, HR 0.29, p = 0.03, treatment 35, control 45, inverted to make HR<1 favor treatment, multivariate.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Cai et al., 18 Mar 2020, retrospective, China, peer-reviewed, 26 authors.
Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
Engineering, doi:10.1016/j.eng.2020.03.007
There is currently an outbreak of respiratory disease caused by a novel coronavirus. The virus has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes has been named coronavirus disease 2019 . More than 16% of patients developed acute respiratory distress syndrome, and the fatality ratio was 1%-2%. No specific treatment has been reported. Herein, we examined the effects of favipiravir (FPV) versus lopinavir (LPV)/ritonavir (RTV) for the treatment of COVID-19. Patients with laboratory-confirmed COVID-19 who received oral FPV (Day 1: 1600 mg twice daily; Days 2-14: 600 mg twice daily) plus interferon (IFN)-a by aerosol inhalation (5 million international unit (IU) twice daily) were included in the FPV arm of this study, whereas patients who were treated with LPV/RTV (Days 1-14: 400 mg/100 mg twice daily) plus IFN-a by aerosol inhalation (5 million IU twice daily) were included in the control arm. Changes in chest computed tomography (CT), viral clearance, and drug safety were compared between the two groups. For the 35 patients enrolled in the FPV arm and the 45 patients in the control arm, all baseline characteristics were comparable between the two arms. A shorter viral clearance median time was found for the FPV arm versus the control arm (4 d (interquartile range (IQR): 2.5-9) versus 11 d (IQR: 8-13), P < 0.001). The FPV arm also showed significant improvement in chest CT compared with the control arm, with an improvement rate of 91.43% versus 62.22% (P = 0.004). After adjustment for potential confounders, the FPV arm also showed a significantly higher improvement rate in chest CT. Multivariable Cox regression showed that FPV was independently associated with faster viral clearance. In addition, fewer adverse events were found in the FPV arm than in the control arm. In this open-label before-after controlled study, FPV showed better therapeutic responses on COVID-19 in terms of disease progression and viral clearance. These preliminary clinical results provide useful information of treatments for SARS-CoV-2 infection.
References
Authors' Contribution Lei, Liu, Liu, Cai, Yang et al., that they have no conflict of interest or financial conflicts to disclose
Bouazza, Treluyer, Foissac, Mentré, Taburet et al., Favipiravir for children with Ebola, Lancet
Chafekar, Fielding, MERS-CoV: understanding the latest human coronavirus threat, Viruses
Chang, Yu, Chang, Galvin, Liu et al., Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT, Radiology
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Grieser, Goldmann, Steffen, Kastrup, Fernández et al., Computed tomography findings from patients with ARDS due to Influenza A (H1N1) virus-associated pneumonia, Eur J Radiol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Li, Guan, Wu, Wang, Zhou et al., Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, N Engl J Med
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet
Madelain, Oestereich, Graw, Nguyen, De Lamballerie et al., Ebola virus dynamics in mice treated with favipiravir, Antiviral Res
Mdvi, Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults [Internet
Oestereich, Lüdtke, Wurr, Rieger, Muñoz-Fontela et al., Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model, Antiviral Res
Sissoko, Laouenan, Folkesson, Lebing, Beavogui et al., Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
DOI record:
{
"DOI": "10.1016/j.eng.2020.03.007",
"ISSN": [
"2095-8099"
],
"URL": "http://dx.doi.org/10.1016/j.eng.2020.03.007",
"alternative-id": [
"S2095809920300631"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Engineering"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eng.2020.03.007"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 THE AUTHORS. Published by Elsevier LTD on behalf of Chinese Academy of Engineering and Higher Education Press Limited Company."
}
],
"author": [
{
"affiliation": [],
"family": "Cai",
"given": "Qingxian",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yang",
"given": "Minghui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Dongjing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shu",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xia",
"given": "Junxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liao",
"given": "Xuejiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gu",
"given": "Yuanbo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cai",
"given": "Qiue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Chenguang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xiaohe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peng",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Deliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shurong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Fuxiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Jiaye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Shuyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Zhaoqin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Zheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Ruiyuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Wu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yingxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Lei",
"sequence": "additional"
}
],
"container-title": "Engineering",
"container-title-short": "Engineering",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
3,
18
]
],
"date-time": "2020-03-18T05:17:07Z",
"timestamp": 1584508627000
},
"deposited": {
"date-parts": [
[
2021,
7,
29
]
],
"date-time": "2021-07-29T19:18:21Z",
"timestamp": 1627586301000
},
"funder": [
{
"DOI": "10.13039/501100018537",
"award": [
"2017ZX10204401",
"2018ZX10711001",
"2017ZX10103011",
"2018ZX09711003",
"2020YFC0841700"
],
"doi-asserted-by": "crossref",
"name": "National Science and Technology Major Project"
},
{
"DOI": "10.13039/501100012151",
"award": [
"SZSM201412003",
"SZSM201512005"
],
"doi-asserted-by": "publisher",
"name": "Sanming Project of Medicine in Shenzhen"
},
{
"DOI": "10.13039/100006190",
"award": [
"202002073000001"
],
"doi-asserted-by": "publisher",
"name": "Shenzhen Science and Technology Research and Development Project"
},
{
"DOI": "10.13039/501100002858",
"award": [
"2019M660836"
],
"doi-asserted-by": "publisher",
"name": "China Postdoctoral Science Foundation"
},
{
"name": "Guangdong Special Fund for Science and Technology Innovation Strategy in 2020"
},
{
"DOI": "10.13039/501100007162",
"award": [
"2020B111105001"
],
"doi-asserted-by": "publisher",
"name": "Department of Science and Technology of Guangdong Province"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T02:18:25Z",
"timestamp": 1712542705027
},
"is-referenced-by-count": 761,
"issue": "10",
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2020,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
3,
17
]
],
"date-time": "2020-03-17T00:00:00Z",
"timestamp": 1584403200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2095809920300631?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2095809920300631?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1192-1198",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.eng.2020.03.007_b0005",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.eng.2020.03.007_b0010",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001316",
"article-title": "Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1199",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eng.2020.03.007_b0015",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.eng.2020.03.007_b0020",
"volume": "395",
"year": "2020"
},
{
"key": "10.1016/j.eng.2020.03.007_b0025",
"unstructured": "National Health Commission of the People’s Republic of China. Daily briefing on novel coronavirus cases in China [Internet]. Beijing: National Health Commission of the People’s Republic of China; c2020 [updated 2020 Mar 12; cited 2020; c2020 Feb 25]. Available form: http://en.nhc.gov.cn/DailyBriefing.html."
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"article-title": "Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "565",
"issue": "10224",
"journal-title": "Lancet",
"key": "10.1016/j.eng.2020.03.007_b0030",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.eng.2020.03.007_b0035",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2014.02.014",
"article-title": "Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model",
"author": "Oestereich",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eng.2020.03.007_b0040",
"volume": "105",
"year": "2014"
},
{
"DOI": "10.1016/j.ejrad.2010.12.085",
"article-title": "Computed tomography findings from patients with ARDS due to Influenza A (H1N1) virus-associated pneumonia",
"author": "Grieser",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "2",
"journal-title": "Eur J Radiol",
"key": "10.1016/j.eng.2020.03.007_b0045",
"volume": "81",
"year": "2012"
},
{
"DOI": "10.1148/radiol.2363040958",
"article-title": "Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "1067",
"issue": "3",
"journal-title": "Radiology",
"key": "10.1016/j.eng.2020.03.007_b0050",
"volume": "236",
"year": "2005"
},
{
"DOI": "10.1016/j.antiviral.2015.08.015",
"article-title": "Ebola virus dynamics in mice treated with favipiravir",
"author": "Madelain",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eng.2020.03.007_b0055",
"volume": "123",
"year": "2015"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"article-title": "Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea",
"author": "Sissoko",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PLoS Med",
"key": "10.1016/j.eng.2020.03.007_b0060",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"issue": "2",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eng.2020.03.007_b0065",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(15)60232-X",
"article-title": "Favipiravir for children with Ebola",
"author": "Bouazza",
"doi-asserted-by": "crossref",
"first-page": "603",
"issue": "9968",
"journal-title": "Lancet",
"key": "10.1016/j.eng.2020.03.007_b0070",
"volume": "385",
"year": "2015"
},
{
"key": "10.1016/j.eng.2020.03.007_b0075",
"unstructured": "MDVI, LLC. Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults [Internet]. Bethesda: National Library of Medicine; [update 2015 Nov 11; cited 2020 Mar 7]. Available from: https://clinicaltrials.gov/ct2/show/NCT02008344."
},
{
"DOI": "10.3390/v10020093",
"article-title": "MERS-CoV: understanding the latest human coronavirus threat",
"author": "Chafekar",
"doi-asserted-by": "crossref",
"first-page": "E93",
"issue": "2",
"journal-title": "Viruses",
"key": "10.1016/j.eng.2020.03.007_b0080",
"volume": "10",
"year": "2018"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.737589209.793575081",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2095809920300631"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Engineering",
"Energy Engineering and Power Technology",
"Materials Science (miscellaneous)",
"General Chemical Engineering",
"Environmental Engineering",
"General Computer Science"
],
"subtitle": [],
"title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "6"
}