Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciab962, Nov 2021
RCT 500 hospitalized patients in Malaysia, showing no significant differences with favipiravir treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
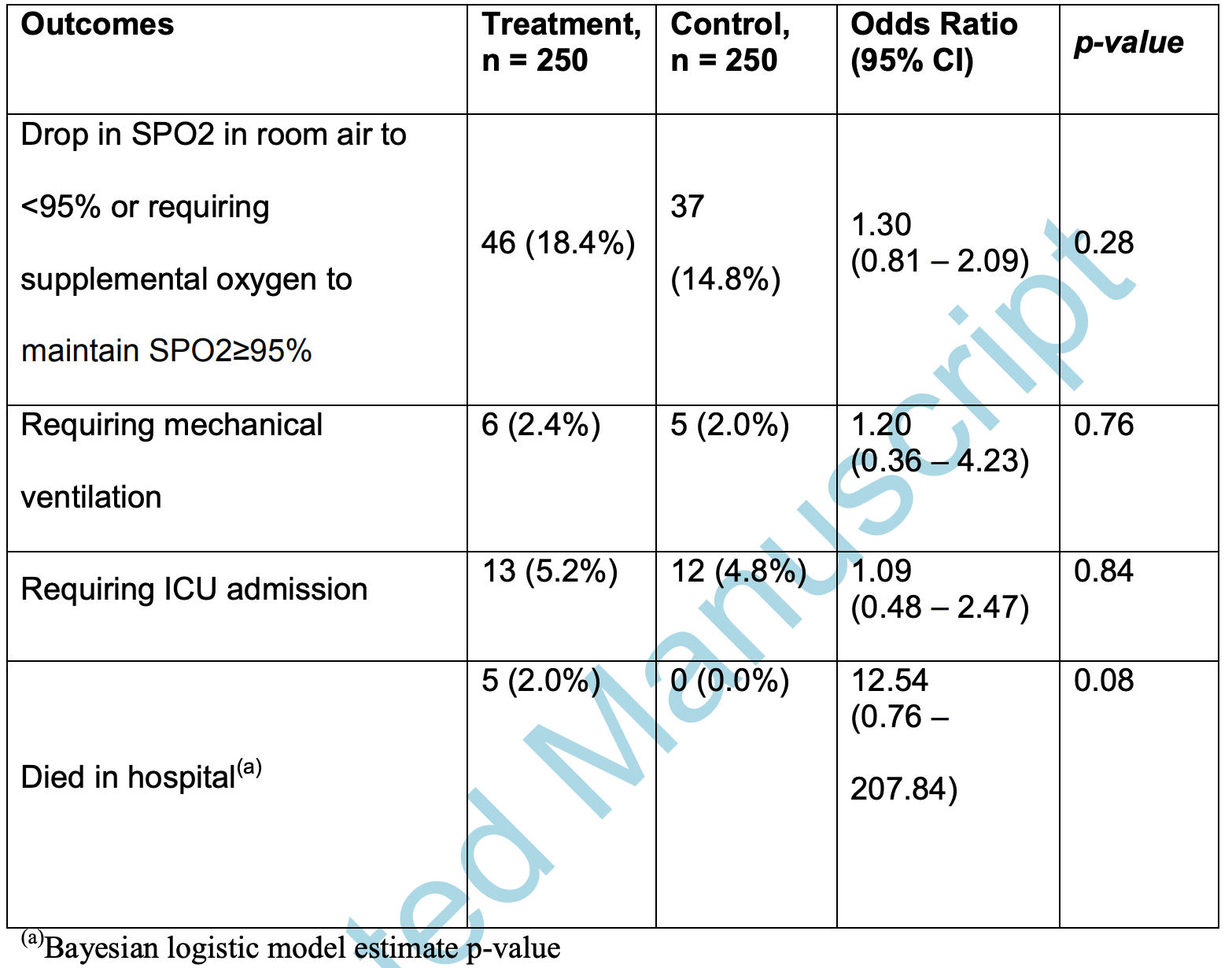
risk of death, 1154.0% higher, RR 12.54, p = 0.08, treatment 5 of 250 (2.0%), control 0 of 250 (0.0%), odds ratio converted to relative risk, continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 19.5% higher, RR 1.20, p = 0.76, treatment 6 of 250 (2.4%), control 5 of 250 (2.0%), odds ratio converted to relative risk.
|
|
risk of ICU admission, 8.5% higher, RR 1.09, p = 0.84, treatment 13 of 250 (5.2%), control 12 of 250 (4.8%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Chuah et al., 19 Nov 2021, Randomized Controlled Trial, Malaysia, peer-reviewed, 18 authors, study period February 2021 - July 2021.
Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial
doi:10.1093/cid/ciab962/6432025
Role of favipiravir remains uncertain in COVID-19. Early treatment with five days oral favipiravir did not prevent disease progression from non-hypoxia to hypoxia in high-risk patients. No beneficial effects were observed in preventing mechanical ventilation, ICU admission, and in-hospital mortality.
A c c e p t e d M a n u s c r i p t 18 moderate to severe diseases. All the recruited patients had risk factors for disease progression and half had evidence of COVID-19 pneumonia. Up to 97.4% of subjects completed the study with outcomes available for evaluation. Patients in intervention group received adequate dosing and duration of favipiravir before the occurrence of clinical deterioration around 4.5 (8.72 ) days from the study enrolment. The use of disease-modifying therapies which could improve clinical outcomes were also equal in both groups.
CONCLUSION Favipiravir did not exert any beneficial effect in preventing clinical deterioration from non-hypoxia to hypoxia among COVID-19 patients with co-morbidity. There was also no effect in reducing incidences of mechanical ventilation, ICU admission, and mortality during hospitalization.
ACKNOWLEDGEMENT The authors would like to thank researchers from all 14 designated COVID-19 public hospitals and Institute for Clinical Research for the contribution in this study. We are grateful of participation from all our patients. We also thank the Director-General of Health Malaysia for his permission to publish this study.
Members of the Malaysian Favipiravir Study
References
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, New England Journal of Medicine
Cai, Yang, Liu, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, The Lancet Microbe
Chen, Zhang, Huang, Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial
Covid, Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk
Dabbous, Abd-Elsalam, El-Sayed, Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study, Arch Virol
Doi, Hibino, Hase, A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother
Du, Chen, Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection
Du, Chen, Response to 'Dose Rationale for Favipiravir Use in Patients Infected With SARS-CoV-2
Eloy, Solas, Touret, Dose Rationale for Favipiravir Use in Patients Infected With SARS-CoV-2
Fajnzylber, Regan, Coxen, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nature Communications
Goyal, Cardozo-Ojeda, Schiffer, Potency and timing of antiviral therapy as determinants of duration of SARS CoV-2 shedding and intensity of inflammatory response, medRxiv
Harris, Taylor, Minor, The REDCap consortium: Building an international community of software platform partners, Journal of Biomedical Informatics
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support, Journal of Biomedical Informatics
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet
Ison, Scheetz, Understanding the pharmacokinetics of Favipiravir: Implications for treatment of influenza and COVID-19, EBioMedicine
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clinical
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Lou, Liu, Yao, Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis
Moh, for data and insights on COVID-19
Naing, Statistics Resources by Lin Naing
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, Journal of Virus Eradication
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, Journal of virus eradication
Saber-Ayad, Saleh, Abu-Gharbieh, The Rationale for Potential Pharmacotherapy of COVID-19, Pharmaceuticals
Seneviratne, Abeysuriya, Mel, Zoysa, Niloofa, Favipiravir in Covid-19
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virology journal
Sim, Chidambaram, Wong, Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study, The Lancet Regional Health -Western Pacific
Sohrabi, Alsafi, Neill, World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int J Surg
Udwadia, Singh, Barkate, Efficacy and Safety of Favipiravir, an Oral RNA-Dependent RNA Polymerase Inhibitor, in Mild-to-Moderate COVID-19: A Randomized, Comparative, Open-Label, Multicenter, Phase 3 Clinical Trial, International Journal of Infectious Diseases
Yu, Feng, Uyeki, Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China, Clin Infect Dis
DOI record:
{
"DOI": "10.1093/cid/ciab962",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciab962",
"container-title": [
"Clinical Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
16
]
],
"date-time": "2021-11-16T20:12:23Z",
"timestamp": 1637093543000
},
"deposited": {
"date-parts": [
[
2021,
11,
18
]
],
"date-time": "2021-11-18T20:14:44Z",
"timestamp": 1637266484000
},
"indexed": {
"date-parts": [
[
2021,
11,
19
]
],
"date-time": "2021-11-19T15:13:55Z",
"timestamp": 1637334835798
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1058-4838"
},
{
"type": "electronic",
"value": "1537-6591"
}
],
"issued": {
"date-parts": [
[
2021
]
]
},
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Clinical Infectious Diseases"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"OUP accepted manuscript"
],
"type": "journal-article"
}