Efficacy and Safety of Andrographolide and Favipiravir Versus Favipiravir Monotherapy in Patients with Mild COVID-19 Infection: A Multicenter Randomized Controlled Trial
et al., OBM Integrative and Complementary Medicine, doi:10.21926/obm.icm.2401013, TCTR20210906002, Feb 2024
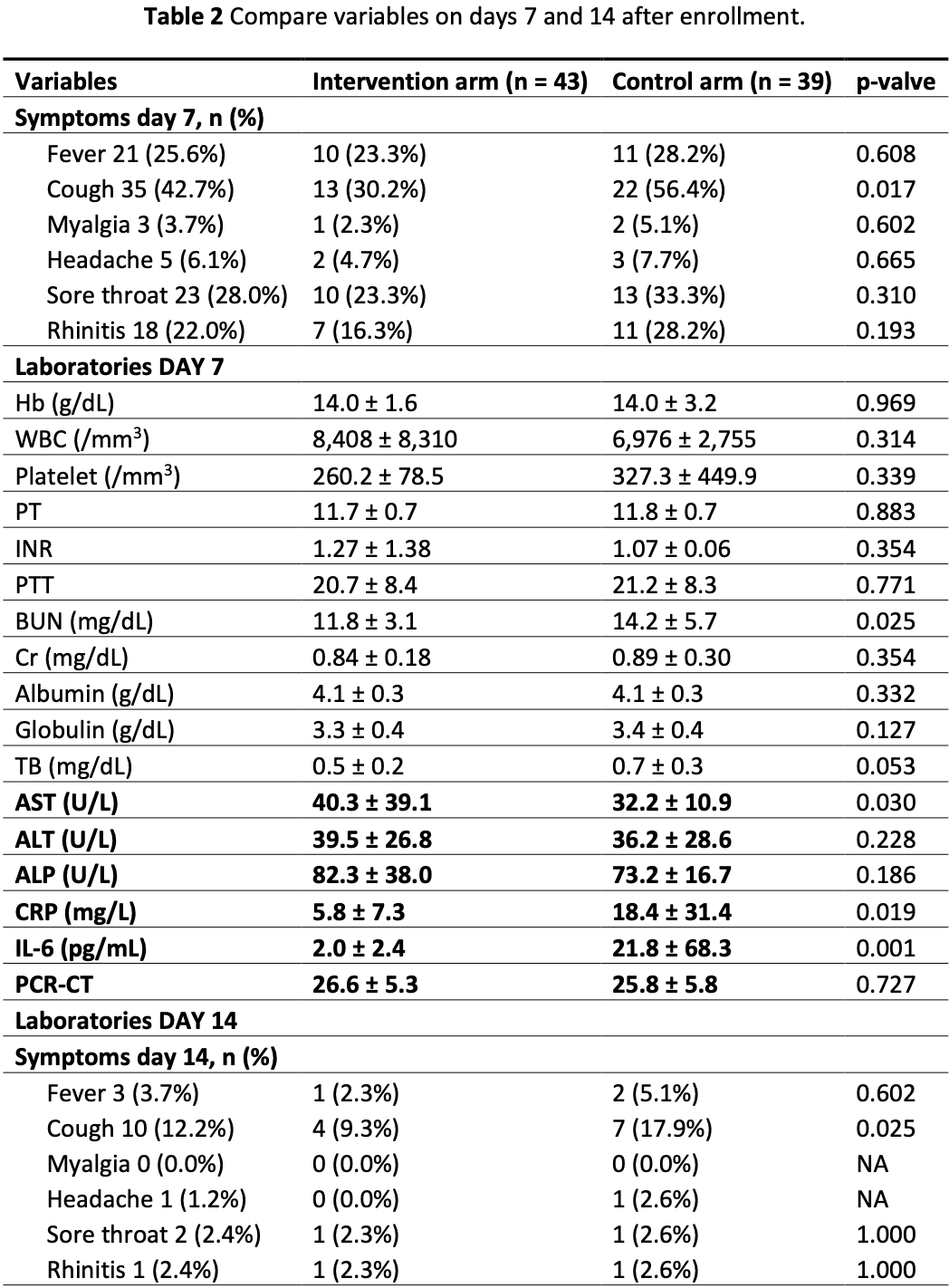
Randomized controlled trial of 82 mild COVID-19 outpatients showing significantly greater reduction in cough and lower inflammatory markers at day 7. Symptomatic improvement was significant at day 7 when combining all symptoms reported, but not for other symptoms individually. There was no progression to severe pneumonia in either group.
|
risk of no recovery, 37.5% lower, RR 0.63, p = 0.005, treatment 43, control 39, all symptoms combined.
|
|
risk of no recovery, 17.5% lower, RR 0.82, p = 0.62, treatment 10 of 43 (23.3%), control 11 of 39 (28.2%), NNT 20, day 7, fever.
|
|
risk of no recovery, 46.4% lower, RR 0.54, p = 0.03, treatment 13 of 43 (30.2%), control 22 of 39 (56.4%), NNT 3.8, day 7, cough.
|
|
risk of no recovery, 54.7% lower, RR 0.45, p = 0.60, treatment 1 of 43 (2.3%), control 2 of 39 (5.1%), NNT 36, day 7, myalgia.
|
|
risk of no recovery, 39.5% lower, RR 0.60, p = 0.66, treatment 2 of 43 (4.7%), control 3 of 39 (7.7%), NNT 33, day 7, headache.
|
|
risk of no recovery, 30.2% lower, RR 0.70, p = 0.34, treatment 10 of 43 (23.3%), control 13 of 39 (33.3%), NNT 9.9, day 7, sore throat.
|
|
risk of no recovery, 42.3% lower, RR 0.58, p = 0.29, treatment 7 of 43 (16.3%), control 11 of 39 (28.2%), NNT 8.4, day 7, rhinitis.
|
|
risk of no recovery, 45.0% lower, RR 0.55, p = 0.18, treatment 43, control 39, all symptoms combined.
|
|
risk of no recovery, 54.7% lower, RR 0.45, p = 0.60, treatment 1 of 43 (2.3%), control 2 of 39 (5.1%), NNT 36, day 14, fever.
|
|
risk of no recovery, 48.2% lower, RR 0.52, p = 0.34, treatment 4 of 43 (9.3%), control 7 of 39 (17.9%), NNT 12, day 14, cough.
|
|
risk of no recovery, 67.8% lower, RR 0.32, p = 0.48, treatment 0 of 43 (0.0%), control 1 of 39 (2.6%), NNT 39, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14, headache.
|
|
risk of no recovery, 9.3% lower, RR 0.91, p = 1.00, treatment 1 of 43 (2.3%), control 1 of 39 (2.6%), NNT 419, day 14, sore throat.
|
|
risk of no recovery, 9.3% lower, RR 0.91, p = 1.00, treatment 1 of 43 (2.3%), control 1 of 39 (2.6%), NNT 419, day 14, rhinitis.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Prasoppokakorn et al., 2 Feb 2024, Randomized Controlled Trial, Thailand, peer-reviewed, 7 authors, study period October 2021 - February 2022, trial TCTR20210906002.
Contact: thanineeeve@gmail.com, supachaya.sr@gmail.com, ercp@live.com, nutbordee@gmail.com, thitagiftr@gmail.com, pongtornmd18@gmail.com, pisittkvn@yahoo.com.
Efficacy and Safety of Andrographolide and Favipiravir Versus Favipiravir Monotherapy in Patients with Mild COVID-19 Infection: A Multicenter Randomized Controlled Trial
OBM Integrative and Complementary Medicine, doi:10.21926/obm.icm.2401013
Reports indicate that Andrographolide inhibits viral replication and reduces COVID-19 symptoms. This study aimed to determine Andrographolide's additional effect and safety in mild COVID-19 patients treated with favipiravir. A multicenter, open-labeled, randomized controlled trial was conducted from October 2021 to February 2022. The patients were randomized to receive a combination of Andrographolide and favipiravir or favipiravir monotherapy. The primary outcome was the occurrence rate of severe pneumonia. The secondary outcomes were symptom improvement, inflammatory biomarkers, and adverse events on days 7 and 14. 82 mild COVID-19 patients were enrolled; 43 and 39 patients received either combination therapy or favipiravir alone. Baseline characteristics were comparable. None developed severe pneumonia, requiring a mechanical ventilator. The Andrographolide group had a significant reduction of cough compared to the controlled group; 13 (30.2%) vs. 22 (56.4%), p = 0.017 on day 7 and 4 (9.3%) vs. 7 (17.9%), p = 0.025 on day 14. Moreover, the Andrographolide group had significantly lower levels of inflammatory markers on day 7, CRP (5.8 vs. 18.4 mg/L; p = 0.019) and IL-6 (2.0 vs. 21.8 pg/mL; p = 0.001) but not on day 14. Regarding safety outcomes, the Andrographolide group had significantly higher AST levels on day 7 (40.3 vs. 32.2 U/L; p = 0.030) and both AST and ALT levels on day 14 (55.3 vs. 32.0; p = 0.014 and 63.8 vs. 40.0; p = 0.022, respectively). In mild COVID-19 patients, the combination of Andrographolide and favipiravir did not demonstrate additional benefits over favipiravir alone in preventing severe pneumonia. However, Andrographolide significantly reduced cough symptoms, especially during the first week. Furthermore, despite mild transaminitis, patients treated with Andrographolide showed improvements in inflammatory markers.
Author Contributions Thaninee Prasoppokakorn, Supachaya Sriphoosanaphan, Rungsun Rerknimitr -Conceptual design of the work; Nutbordee Nalinthassanai, Thitaporn Roongrawee, Pongtorn Hanboonkunupakarn, Pisit Tangkijvanich -Data collection and data acquisition; Thaninee Prasoppokakorn, Supachaya Sriphoosanaphan, Rungsun Rerknimitr -Data analysis and interpretation; Thaninee Prasoppokakorn -Drafting the manuscript; Supachaya Sriphoosanaphan, Rungsun Rerknimitr -Critical revision of the manuscript; All authors -Final approval of the version to be published.
Competing Interests The authors declare no conflicts of interests.
References
Akbar, Andrographis paniculata: A review of pharmacological activities and clinical effects, Altern Med Rev
Damiati, Bahlas, Aljohaney, Bawazir, Mustafa et al., Implications of SARS-CoV-2 infection on the clinical, hematological, and inflammatory parameters in COVID-19 patients: A retrospective cross-sectional study, J Infect Public Health
Das, Das, Swain, Mukherjee, Bhattacharya, Andrographolide induces anti-SARS-CoV-2 response through host-directed mechanism: An in silico study, Future Virol
Ergür, Yıldız, Şener, Kavurgacı, Ozturk, Adverse effects associated with favipiravir in patients with COVID-19 pneumonia: A retrospective study, Sao Paulo Med J
Ferreira, Polonini, Dijkers, Postulated adjuvant therapeutic strategies for COVID-19, J Pers Med
Guérin, Mclean, Rashan, Lawal, Watson et al., Definitions matter: Heterogeneity of COVID-19 disease severity criteria and incomplete reporting compromise meta-analysis, PLoS Glob Public Health
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of favipiravir in treatment of COVID-19: A systematic review and metaanalysis of clinical trials, Sci Rep
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Li, Yuan, Wu, Zhen, Sun et al., Andrographolide, a natural anti-inflammatory agent: An update, Front Pharmacol
Nguyen, Do, Phan, Huynh, The potential of ameliorating COVID-19 and sequelae from andrographis paniculata via bioinformatics, Bioinform Biol Insights, doi:10.1177/11779322221149622
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol
Qin, Kong, Shi, Wang, Ge, Andrographolide inhibits the production of TNF-α and interleukin-12 in lipopolysaccharide-stimulated macrophages: Role of mitogen-activated protein kinases, Biol Pharm Bull
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Santos, Natural history of COVID-19 and current knowledge on treatment therapeutic options, Biomed Pharmacother
Shi, Huang, Chen, Pi, Hsu et al., Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage, Biochem Biophys Res Commun
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of favipiravir in COVID-19 patients without pneumonia: A multicentre, openlabelled, randomized control study, Emerg Microbes Infect
Siripongboonsitti, Ungtrakul, Tawinprai, Auewarakul, Chartisathian et al., Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial), Phytomedicine
Srikanth, Sarma, Andrographolide binds to spike glycoprotein and RNA-dependent RNA polymerase (NSP12) of SARS-CoV-2 by in silico approach: A probable molecule in the development of anti-coronaviral drug, J Genet Eng Biotechnol
Statsenko, Zahmi, Habuza, Almansoori, Smetanina et al., Impact of age and sex on COVID-19 severity assessed from radiologic and clinical findings, Front Cell Infect Microbiol
Vieillard-Baron, Flicoteaux, Salmona, Annane, Ayed et al., Epidemiological characteristics and severity of omicron variant cases in the APHP critical care units, MedRxiv, doi:10.1101/2022.01.25.22269839
Zhang, Lv, Zhou, Xie, Xu et al., Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial, Phytother Res
Zhu, Hou, Yang, Network pharmacology integrated with experimental validation revealed the anti-inflammatory effects of Andrographis paniculata, Sci Rep
DOI record:
{
"DOI": "10.21926/obm.icm.2401013",
"ISSN": [
"2573-4393"
],
"URL": "http://dx.doi.org/10.21926/obm.icm.2401013",
"abstract": "<jats:p>Reports indicate that Andrographolide inhibits viral replication and reduces COVID-19 symptoms. This study aimed to determine Andrographolide's additional effect and safety in mild COVID-19 patients treated with favipiravir. A multicenter, open-labeled, randomized controlled trial was conducted from October 2021 to February 2022. The patients were randomized to receive a combination of Andrographolide and favipiravir or favipiravir monotherapy. The primary outcome was the occurrence rate of severe pneumonia. The secondary outcomes were symptom improvement, inflammatory biomarkers, and adverse events on days 7 and 14. 82 mild COVID-19 patients were enrolled; 43 and 39 patients received either combination therapy or favipiravir alone. Baseline characteristics were comparable. None developed severe pneumonia, requiring a mechanical ventilator. The Andrographolide group had a significant reduction of cough compared to the controlled group; 13 (30.2%) vs. 22 (56.4%), p = 0.017 on day 7 and 4 (9.3%) vs. 7 (17.9%), p = 0.025 on day 14. Moreover, the Andrographolide group had significantly lower levels of inflammatory markers on day 7, CRP (5.8 vs. 18.4 mg/L; p = 0.019) and IL-6 (2.0 vs. 21.8 pg/mL; p = 0.001) but not on day 14. Regarding safety outcomes, the Andrographolide group had significantly higher AST levels on day 7 (40.3 vs. 32.2 U/L; p = 0.030) and both AST and ALT levels on day 14 (55.3 vs. 32.0; p = 0.014 and 63.8 vs. 40.0; p = 0.022, respectively). In mild COVID-19 patients, the combination of Andrographolide and favipiravir did not demonstrate additional benefits over favipiravir alone in preventing severe pneumonia. However, Andrographolide significantly reduced cough symptoms, especially during the first week. Furthermore, despite mild transaminitis, patients treated with Andrographolide showed improvements in inflammatory markers.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Prasoppokakorn",
"given": "Thaninee",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sriphoosanaphan",
"given": "Supachaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nalinthassanai",
"given": "Nutbordee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roongrawee",
"given": "Thitaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Pongtorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tangkijvanich",
"given": "Pisit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rerknimitr",
"given": "Rungsun",
"sequence": "additional"
}
],
"container-title": "OBM Integrative and Complementary Medicine",
"container-title-short": "OBM Integr Complement Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
2
]
],
"date-time": "2024-02-02T08:29:15Z",
"timestamp": 1706862555000
},
"deposited": {
"date-parts": [
[
2024,
2,
2
]
],
"date-time": "2024-02-02T08:29:41Z",
"timestamp": 1706862581000
},
"indexed": {
"date-parts": [
[
2024,
2,
3
]
],
"date-time": "2024-02-03T00:29:37Z",
"timestamp": 1706920177082
},
"is-referenced-by-count": 0,
"issue": "01",
"issued": {
"date-parts": [
[
2024,
2,
2
]
]
},
"journal-issue": {
"issue": "01",
"published-online": {
"date-parts": [
[
2024
]
]
},
"published-print": {
"date-parts": [
[
2024
]
]
}
},
"member": "9387",
"original-title": [],
"page": "1-17",
"prefix": "10.21926",
"published": {
"date-parts": [
[
2024,
2,
2
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
2
]
]
},
"published-print": {
"date-parts": [
[
2024,
2,
2
]
]
},
"publisher": "LIDSEN Publishing Inc",
"reference": [
{
"DOI": "10.1038/s41598-021-90551-6",
"doi-asserted-by": "crossref",
"key": "ref=1",
"unstructured": "Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials. Sci Rep. 2021; 11: 11022."
},
{
"DOI": "10.1080/22221751.2022.2117092",
"doi-asserted-by": "crossref",
"key": "ref=2",
"unstructured": "Sirijatuphat R, Manosuthi W, Niyomnaitham S, Owen A, Copeland KK, Charoenpong L, et al. Early treatment of favipiravir in COVID-19 patients without pneumonia: A multicentre, open-labelled, randomized control study. Emerg Microbes Infect. 2022; 11: 2197-2206."
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"doi-asserted-by": "crossref",
"key": "ref=3",
"unstructured": "Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021; 102: 501-508."
},
{
"DOI": "10.1101/2022.01.25.22269839",
"doi-asserted-by": "crossref",
"key": "ref=4",
"unstructured": "Vieillard-Baron A, Flicoteaux R, Salmona M, Annane D, Ayed S, Azoulay E, et al. Epidemiological characteristics and severity of omicron variant cases in the APHP critical care units. MedRxiv. 2022. doi: 10.1101/2022.01.25.22269839."
},
{
"DOI": "10.1248/bpb.29.220",
"doi-asserted-by": "crossref",
"key": "ref=5",
"unstructured": "Qin LH, Kong L, Shi GJ, Wang ZT, Ge BX. Andrographolide inhibits the production of TNF-α and interleukin-12 in lipopolysaccharide-stimulated macrophages: Role of mitogen-activated protein kinases. Biol Pharm Bull. 2006; 29: 220-224."
},
{
"key": "ref=6",
"unstructured": "Akbar S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern Med Rev. 2011; 16: 66-77."
},
{
"DOI": "10.1177/11779322221149622",
"doi-asserted-by": "crossref",
"key": "ref=7",
"unstructured": "Nguyen HT, Do VM, Phan TT, Nguyen Huynh DT. The potential of ameliorating COVID-19 and sequelae from andrographis paniculata via bioinformatics. Bioinform Biol Insights. 2023; 17. doi: 10.1177/11779322221149622."
},
{
"DOI": "10.3389/fphar.2022.920435",
"doi-asserted-by": "crossref",
"key": "ref=8",
"unstructured": "Li X, Yuan W, Wu J, Zhen J, Sun Q, Yu M. Andrographolide, a natural anti-inflammatory agent: An update. Front Pharmacol. 2022; 13: 920435."
},
{
"DOI": "10.1038/s41598-021-89257-6",
"doi-asserted-by": "crossref",
"key": "ref=9",
"unstructured": "Zhu N, Hou J, Yang N. Network pharmacology integrated with experimental validation revealed the anti-inflammatory effects of Andrographis paniculata. Sci Rep. 2021; 11: 9752."
},
{
"DOI": "10.2217/fvl-2021-0171",
"doi-asserted-by": "crossref",
"key": "ref=10",
"unstructured": "Das BS, Das NC, Swain SS, Mukherjee S, Bhattacharya D. Andrographolide induces anti-SARS-CoV-2 response through host-directed mechanism: An in silico study. Future Virol. 2022; 17: 651-673."
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"doi-asserted-by": "crossref",
"key": "ref=11",
"unstructured": "Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, Kanjanasirirat P, Manopwisedjaroen S, et al. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021; 84: 1261-1270."
},
{
"DOI": "10.1186/s43141-021-00201-7",
"doi-asserted-by": "crossref",
"key": "ref=12",
"unstructured": "Srikanth L, Sarma PV. Andrographolide binds to spike glycoprotein and RNA-dependent RNA polymerase (NSP12) of SARS-CoV-2 by in silico approach: A probable molecule in the development of anti-coronaviral drug. J Genet Eng Biotechnol. 2021; 19: 101."
},
{
"DOI": "10.1016/j.bbrc.2020.08.086",
"doi-asserted-by": "crossref",
"key": "ref=13",
"unstructured": "Shi TH, Huang YL, Chen CC, Pi WC, Hsu YL, Lo LC, et al. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem Biophys Res Commun. 2020; 533: 467-473."
},
{
"DOI": "10.1002/ptr.7141",
"doi-asserted-by": "crossref",
"key": "ref=14",
"unstructured": "Zhang XY, Lv L, Zhou YL, Xie LD, Xu Q, Zou XF, et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother Res. 2021; 35: 4401-4410."
},
{
"DOI": "10.1016/j.phymed.2023.155018",
"doi-asserted-by": "crossref",
"key": "ref=15",
"unstructured": "Siripongboonsitti T, Ungtrakul T, Tawinprai K, Auewarakul C, Chartisathian W, Jansala T, et al. Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial). Phytomedicine. 2023; 119: 155018."
},
{
"key": "ref=16",
"unstructured": "National Institutes of Health (NIH). Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. Bethesda, MD: National Institutes of Health (NIH); 2023. Available from: https://www.covid19treatmentguidelines.nih.gov/."
},
{
"DOI": "10.1371/journal.pgph.0000561",
"doi-asserted-by": "crossref",
"key": "ref=17",
"unstructured": "Guérin PJ, McLean AR, Rashan S, Lawal A, Watson JA, Strub-Wourgaft N, et al. Definitions matter: Heterogeneity of COVID-19 disease severity criteria and incomplete reporting compromise meta-analysis. PLoS Glob Public Health. 2022; 2: e0000561."
},
{
"key": "ref=18",
"unstructured": "Centers for Disease Control and Prevention. Underlying medical conditions [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2023. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html."
},
{
"DOI": "10.1016/j.biopha.2020.110493",
"doi-asserted-by": "crossref",
"key": "ref=19",
"unstructured": "Dos Santos WG. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. 2020; 129: 110493."
},
{
"DOI": "10.3390/jpm10030080",
"doi-asserted-by": "crossref",
"key": "ref=20",
"unstructured": "Ferreira AO, Polonini HC, Dijkers EC. Postulated adjuvant therapeutic strategies for COVID-19. J Pers Med. 2020; 10: 80."
},
{
"DOI": "10.1038/s41579-022-00822-w",
"doi-asserted-by": "crossref",
"key": "ref=21",
"unstructured": "Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023; 21: 147-161."
},
{
"DOI": "10.3389/fcimb.2021.777070",
"doi-asserted-by": "crossref",
"key": "ref=22",
"unstructured": "Statsenko Y, Al Zahmi F, Habuza T, Almansoori TM, Smetanina D, Simiyu GL, et al. Impact of age and sex on COVID-19 severity assessed from radiologic and clinical findings. Front Cell Infect Microbiol. 2021; 11: 777070."
},
{
"DOI": "10.1016/j.jiph.2021.12.013",
"doi-asserted-by": "crossref",
"key": "ref=23",
"unstructured": "Damiati LA, Bahlas S, Aljohaney A, Bawazir Y, Mustafa M, Denetiu I, et al. Implications of SARS-CoV-2 infection on the clinical, hematological, and inflammatory parameters in COVID-19 patients: A retrospective cross-sectional study. J Infect Public Health. 2022; 15: 214-221."
},
{
"DOI": "10.1590/1516-3180.2021.0489.r1.13082021",
"doi-asserted-by": "crossref",
"key": "ref=24",
"unstructured": "Ergür FÖ, Yıldız M, Şener MU, Kavurgacı S, Ozturk A. Adverse effects associated with favipiravir in patients with COVID-19 pneumonia: A retrospective study. Sao Paulo Med J. 2022; 140: 372-377."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.lidsen.com/journals/icm/icm-09-01-013"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy and Safety of Andrographolide and Favipiravir Versus Favipiravir Monotherapy in Patients with Mild COVID-19 Infection: A Multicenter Randomized Controlled Trial",
"type": "journal-article",
"volume": "09"
}