Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19
et al., NEJM, doi:10.1056/NEJMoa2201662, COVID-OUT, NCT04510194, Aug 2022
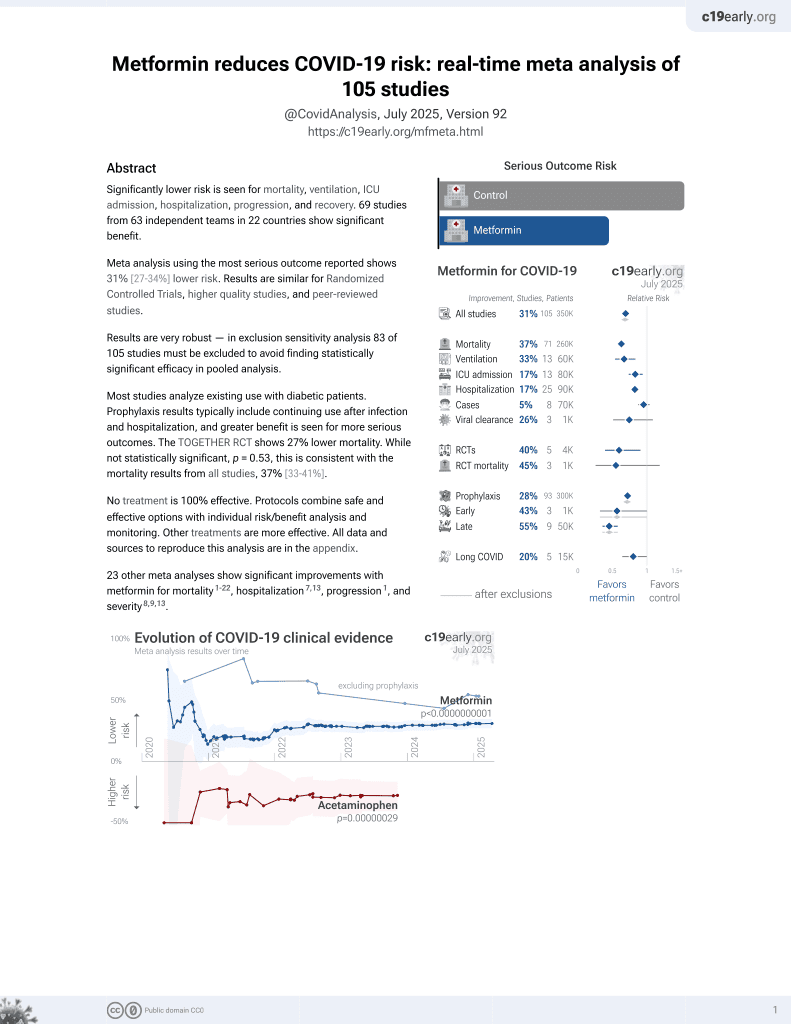
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
COVID-OUT remotely operated RCT, showing lower
combined ER/hospitalization/death with metformin. Results for other treatments are listed
separately -
ivermectin,
fluvoxamine.
The "control" group includes patients receiving active
treatments fluvoxamine and ivermectin.
Control arm results are very different between treatments, for
example considering hospitalization/death, this was 1.0% for ivermectin vs.
2.7% for overall control, however it was 1.3% for the ivermectin-specific
control. 394 control patients are shared. The rate for the non-shared 261
metformin control patients is 5%, compared to 1.3% for ivermectin control
patients. The metformin arm started earlier, however it is unclear why the
difference in outcomes is so large.
Results were delayed for 6 months with no explanation, with
followup ending Feb 14, 2022.
Adherence was very low, with 77% overall reporting 70+%
adherence. Numbers for 100% adherence are not provided.
Multiple outcomes are missing, for example time to recovery
(where ACTIV-6 showed superiority of ivermectin).
Treatment was 14 days for metformin and fluvoxamine, but only 3
days for ivermectin.
Medication delivery varied significantly over the trial. In
this presentation3, author indicates that
delivery was initially local, later via FedEx, was much slower in August,
there were delays due to team bandwidth issues, and they only realized they
could use FedEx same day delivery in September.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments4.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers ivermectin, fluvoxamine, and metformin.
|
risk of death, 2.9% lower, RR 0.97, p = 1.00, treatment 1 of 408 (0.2%), control 1 of 396 (0.3%), NNT 13464, day 28.
|
|
risk of death, 197.1% higher, RR 2.97, p = 1.00, treatment 1 of 408 (0.2%), control 0 of 396 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
|
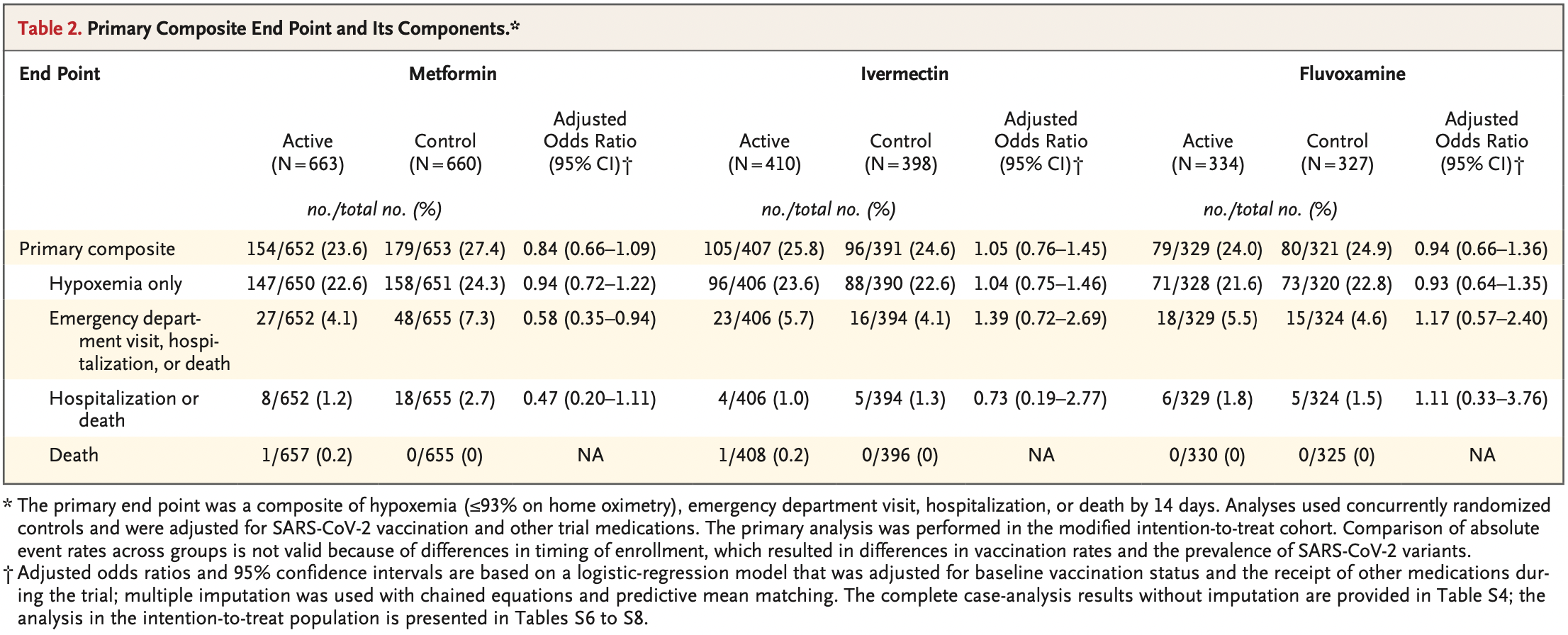
risk of death/hospitalization, 52.3% lower, RR 0.48, p = 0.09, treatment 8 of 652 (1.2%), control 18 of 655 (2.7%), NNT 66, odds ratio converted to relative risk.
|
|
risk of progression, 40.2% lower, RR 0.60, p = 0.03, treatment 27 of 652 (4.1%), control 48 of 655 (7.3%), NNT 31, odds ratio converted to relative risk, combined ER, hospitalization, death.
|
|
risk of progression, 12.1% lower, RR 0.88, p = 0.18, treatment 154 of 652 (23.6%), control 179 of 653 (27.4%), NNT 26, odds ratio converted to relative risk, combined hypoxemia, ER, hospitalization, death, primary outcome.
|
|
risk of no viral clearance, 36.9% lower, RR 0.63, p < 0.001, treatment 72 of 504 (14.3%), control 112 of 495 (22.6%), NNT 12, day 10.
|
|
risk of no viral clearance, 8.7% lower, RR 0.91, p = 0.15, treatment 251 of 504 (49.8%), control 270 of 495 (54.5%), NNT 21, day 5.
|
|
risk of long COVID, 20.9% lower, RR 0.79, p = 0.34, treatment 1,439, control 1,544, adjusted per study, PASCD, day 180.
|
|
risk of long COVID, 50.5% lower, RR 0.49, p = 0.05, treatment 1,439, control 1,544, CDLC, day 180.
|
|
risk of long COVID, 14.0% lower, RR 0.86, p = 0.54, treatment 1,439, control 1,544, symptom burden, day 180.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bramante et al., 18 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 37 authors, average treatment delay 4.8 days, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04510194 (history) (COVID-OUT).
Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2201662
BACKGROUND Early treatment to prevent severe coronavirus disease 2019 (Covid-19) is an important component of the comprehensive response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic.
METHODS In this phase 3, double-blind, randomized, placebo-controlled trial, we used a 2-by-3 factorial design to test the effectiveness of three repurposed drugs -metformin, ivermectin, and fluvoxamine -in preventing serious SARS-CoV-2 infection in nonhospitalized adults who had been enrolled within 3 days after a confirmed diagnosis of infection and less than 7 days after the onset of symptoms. The patients were between the ages of 30 and 85 years, and all had either overweight or obesity. The primary composite end point was hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death. All analyses used controls who had undergone concurrent randomization and were adjusted for SARS-CoV-2 vaccination and receipt of other trial medications.
RESULTS A total of 1431 patients underwent randomization; of these patients, 1323 were included in the primary analysis. The median age of the patients was 46 years; 56% were female (6% of whom were pregnant), and 52% had been vaccinated. The adjusted odds ratio for a primary event was 0.84 (95% confidence interval [CI], 0.66 to 1.09; P = 0.19) with metformin, 1.05 (95% CI, 0.76 to 1.45; P = 0.78) with ivermectin, and 0.94 (95% CI, 0.66 to 1.36; P = 0.75) with fluvoxamine. In prespecified secondary analyses, the adjusted odds ratio for emergency department visit, hospitalization, or death was 0.58 (95% CI, 0.35 to 0.94) with metformin, 1.39 (95% CI, 0.72 to 2.69) with ivermectin, and 1.17 (95% CI, 0.57 to 2.40) with fluvoxamine. The adjusted odds ratio for hospitalization or death was 0.47 (95% CI, 0.20 to 1.11) with metformin, 0.73 (95% CI, 0.19 to 2.77) with ivermectin, and 1.11 (95% CI, 0.33 to 3.76) with fluvoxamine.
CONCLUSIONS None of the three medications that were evaluated prevented the occurrence of hypoxemia, an emergency department visit, hospitalization, or death associated with Covid-19. (Funded by the Parsemus Foundation and others; COVID-OUT ClinicalTrials .gov number, NCT04510194.
Appendix The authors' full names and academic degrees are as follows: Carolyn T. Bramante
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Blitzer, Ham, Colby, Skondra, Association of metformin use with age-related macular degeneration: a casecontrol study, JAMA Ophthalmol
Bolo, Hodé, Nédélec, Lainé, Wagner et al., Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy, Neuropsychopharmacology
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19
Bramante, Buse, Tamaritz, Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity, J Med Virol
Bramante, Ingraham, Murray, Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis, Lancet Healthy Longev
Bramante, Proper, Boulware, Vaccination against SARS-CoV-2 is associated with a lower viral load and Metformin, Ivermectin, and Fluvoxamine for Covid-19 likelihood of systemic symptoms, Open Forum Infect Dis
Campo, García-Valdecasas, Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy, PLoS One
Cariou, Hadjadj, Wargny, Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study, Diabetologia
Castle, Dock, Hemmat, Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets, doi:10.1101/2020.05.22.111237v2
Cheng, He, Jung, Lu, Gao, Suppression of Kaposi's sarcoma-associated herpesvirus infection and replication by 5′-AMP-activated protein kinase, J Virol
Connors, Brooks, Sciurba, Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial, JAMA
Crouse, Grimes, Li, Might, Ovalle et al., Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes, Front Endocrinol
Dodds, Doyle, Reiersen, Brown, Rayner, Fluvoxamine for the treatment of COVID-19, Lancet Glob Health
Facente, Reiersen, Lenze, Boulware, Klausner, Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence, Drugs
Farfan-Morales, Cordero-Rivera, Anti-flavivirus properties of lipid-lowering drugs, Front Physiol
Ferdinands, Rao, Dixon, Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance -VISION Network, 10 states, August 2021, MMWR Morb Mortal Wkly Rep
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Hales, Carroll, Fryar, Ogden, Prevalence of obesity and severe obesity among adults: United States, 2017-2018, NCHS Data Brief
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for highrisk, nonhospitalized adults with Covid-19
Karam, Morris, Bramante, mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses, J Med Virol
Keehner, Horton, Binkin, Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce, N Engl J Med
Lee, Vigod, Bortolussi-Courval, Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis, JAMA Netw Open
Lenze, Mattar, Zorumski, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Li, Yang, Yan, Sun, Zeng et al., Metformin in patients with COVID-19: a systematic review and meta-analysis, Front Med
Luo, Qiu, Liu, Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis, Am J Trop Med Hyg
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA
Marzolini, Kuritzkes, Marra, Prescribing nirmatrelvir-ritonavir: how to recognize and manage drug-drug interactions, Ann Intern Med
Medical, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA
Meng, Db, Performing likelihood ratio tests with multiply-imputed data sets, Biometrika
Nakashima, Takeuchi, Chihara, Hotta, Sada, Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways, Microbiol Immunol
Newberry, Williams, Stauffer, Boulware, Hendel-Paterson et al., Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis, Chest
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Oldenburg, Pinsky, Brogdon, Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial, JAMA
Popp, Stegemann, Metzendorf, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev
Portmann-Baracco, Alberti, Accinelli, Antiviral and anti-inflammatory properties of ivermectin and its potential use in COVID-19, Arch Bronconeumol (Engl Ed)
Postler, Peng, Bhatt, Ghosh, Metformin selectively dampens the acute inflammatory response through an AMPK-dependent mechanism, Sci Rep
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health
Reis, Santos, Silva, Silva, Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial, Lancet Reg Health Am
Schaller, Sharma, Dupee, Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses, JCI Insight
Seftel, Boulware, Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19, Open Forum Infect Dis
Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Intern Med
Stauffer, Alpern, Walker, COVID-19 and dexamethasone: a potential strategy to avoid steroid-related strongyloides hyperinfection, JAMA
Thompson, Natarajan, Irving, Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance -VISION Network, 10 states, August 2021, MMWR Morb Mortal Wkly Rep
Timmins, Donahue, Meeker, Marathe, Steady-state pharmacokinetics of a novel extended-release metformin formulation, Clin Pharmacokinet
Xian, Liu, Nilsson, Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation, Immunity
Xin, Wei, Ji, Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release, Sci Rep
Yu, Sun, Zhao, Kang, Yan, The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance, Int J Infect Dis
DOI record:
{
"DOI": "10.1056/nejmoa2201662",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2201662",
"alternative-id": [
"10.1056/NEJMoa2201662"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5858-2080",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Huling",
"given": "Jared D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Tignanelli",
"given": "Christopher J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9723-3876",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Buse",
"given": "John B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Liebovitz",
"given": "David M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Nicklas",
"given": "Jacinda M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Cohen",
"given": "Kenneth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6358-4670",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Puskarich",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Belani",
"given": "Hrishikesh K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6037-4077",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Proper",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Siegel",
"given": "Lianne K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Klatt",
"given": "Nichole R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7731-2799",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Odde",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Luke",
"given": "Darlette G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Anderson",
"given": "Blake",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2781-3824",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Karger",
"given": "Amy B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0292-0594",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Ingraham",
"given": "Nicholas E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6723-0423",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Hartman",
"given": "Katrina M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Rao",
"given": "Via",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Hagen",
"given": "Aubrey A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Patel",
"given": "Barkha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Fenno",
"given": "Sarah L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Avula",
"given": "Nandini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Reddy",
"given": "Neha V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Erickson",
"given": "Spencer M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Lindberg",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Fricton",
"given": "Regina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Lee",
"given": "Samuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6884-7748",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Zaman",
"given": "Adnin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Saveraid",
"given": "Hanna G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Tordsen",
"given": "Walker J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Pullen",
"given": "Matthew F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Biros",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Sherwood",
"given": "Nancy E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Thompson",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Murray",
"given": "Thomas A.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
17
]
],
"date-time": "2022-08-17T21:07:21Z",
"timestamp": 1660770441000
},
"deposited": {
"date-parts": [
[
2024,
3,
26
]
],
"date-time": "2024-03-26T22:20:11Z",
"timestamp": 1711491611000
},
"funder": [
{
"name": "UnitedHealth Group Foundation"
},
{
"DOI": "10.13039/100016127",
"doi-asserted-by": "publisher",
"name": "Fast Grants"
},
{
"DOI": "10.13039/100006108",
"award": [
"KL2TR002492",
"UL1TR002494"
],
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
},
{
"DOI": "10.13039/100000062",
"award": [
"DK124654-01A1"
],
"doi-asserted-by": "publisher",
"name": "National Institute of Diabetes and Digestive and Kidney Diseases"
},
{
"DOI": "10.13039/100016608",
"doi-asserted-by": "publisher",
"name": "Rainwater Charitable Foundation"
},
{
"name": "The Parsemus Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T20:31:04Z",
"timestamp": 1712608264384
},
"is-referenced-by-count": 139,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
8,
18
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2022,
8,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
18
]
],
"date-time": "2022-08-18T00:00:00Z",
"timestamp": 1660780800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2201662",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "599-610",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
8,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
18
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMc2112981",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_2_2"
},
{
"DOI": "10.15585/mmwr.mm7107e2",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.15585/mmwr.mm7104e3",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1056/NEJMoa2109682",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.7326/M22-0281",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1101/2020.05.22.111237",
"doi-asserted-by": "crossref",
"key": "e_1_3_4_9_2",
"unstructured": "Castle BT Dock C Hemmat M et al. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. June 16 2020 (https://www.biorxiv.org/content/10.1101/2020.05.22.111237v2). preprint."
},
{
"DOI": "10.1002/jmv.26728",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2"
},
{
"DOI": "10.3389/fphys.2021.749770",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.1128/JVI.00624-16",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1371/journal.pone.0191805",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1111/j.1348-0421.2011.00382.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1172/jci.insight.148003",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1038/s41598-021-97441-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1038/srep36222",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1016/j.immuni.2021.05.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1002/jmv.26873",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.4269/ajtmh.20-0375",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1007/s00125-020-05180-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.3389/fmed.2021.704666",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
},
{
"DOI": "10.3389/fendo.2020.600439",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_2"
},
{
"DOI": "10.1016/S2666-7568(20)30033-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_25_2"
},
{
"DOI": "10.1016/j.arbr.2020.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_2"
},
{
"article-title": "Ivermectin for preventing and treating COVID-19.",
"author": "Popp M",
"first-page": "CD015017",
"issue": "7",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_3_4_27_2",
"unstructured": "Popp M, Stegemann M, Metzendorf M-I, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021;7(7):CD015017-CD015017.34318930",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_28_2"
},
{
"DOI": "10.1007/s40265-021-01636-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_2"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_30_2"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_31_2"
},
{
"DOI": "10.1001/jama.2020.22760",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_32_2"
},
{
"DOI": "10.1093/ofid/ofab050",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_33_2"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_34_2"
},
{
"key": "e_1_3_4_35_2",
"unstructured": "Food and Drug Administration. Pulse oximeter accuracy and limitations: FDA safety communication. February 19 2021 (https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication)."
},
{
"key": "e_1_3_4_36_2",
"unstructured": "Food and Drug Administration. Multiple endpoints in clinical trials: guidance for industry. January 2017 (https://www.fda.gov/media/102657/download)."
},
{
"key": "e_1_3_4_37_2",
"unstructured": "Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. September 2020 (https://www.fda.gov/media/142143/download)."
},
{
"key": "e_1_3_4_38_2",
"unstructured": "Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. FDA news release. February 11 2022 (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-monoclonal-antibody-treatment-covid-19-retains)."
},
{
"DOI": "10.1093/ofid/ofac066",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_39_2"
},
{
"DOI": "10.1001/jama.2013.281053",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_40_2"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_41_2"
},
{
"DOI": "10.1001/jama.2021.17272",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_42_2"
},
{
"DOI": "10.1093/biomet/79.1.103",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_43_2"
},
{
"article-title": "Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial.",
"author": "Reis G",
"first-page": "100142",
"journal-title": "Lancet Reg Health Am",
"key": "e_1_3_4_44_2",
"unstructured": "Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022;6:100142-100142.34927127",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1001/jamaophthalmol.2020.6331",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_45_2"
},
{
"DOI": "10.2165/00003088-200544070-00004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_46_2"
},
{
"DOI": "10.1016/S0893-133X(00)00116-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_47_2"
},
{
"DOI": "10.1016/S2214-109X(22)00006-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_48_2"
},
{
"DOI": "10.1378/chest.128.5.3681",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_49_2"
},
{
"DOI": "10.1001/jama.2020.13170",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_50_2"
},
{
"DOI": "10.1016/j.ijid.2012.02.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_51_2"
},
{
"article-title": "Prevalence of obesity and severe obesity among adults: United States, 2017-2018.",
"author": "Hales CM",
"first-page": "1",
"journal-title": "NCHS Data Brief",
"key": "e_1_3_4_52_2",
"unstructured": "Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;360:1-8.32487284",
"volume": "360",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11517",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_53_2"
}
],
"reference-count": 52,
"references-count": 52,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.742308258.793595134",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2201662"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19",
"type": "journal-article",
"volume": "387"
}
covidout