Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19
et al., NEJM, doi:10.1056/NEJMoa2201662, COVID-OUT, NCT04510194, Aug 2022
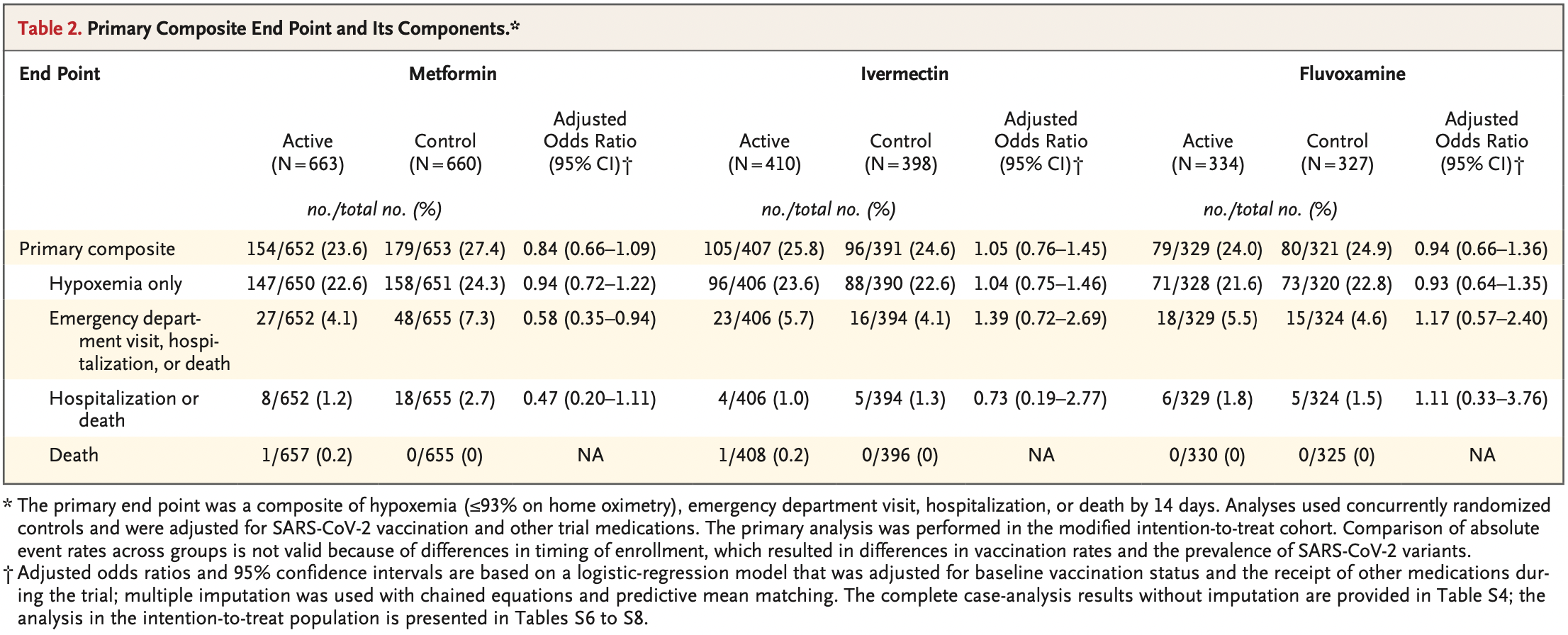
COVID-OUT remote RCT, showing no significant differences
compared to a combined metformin/placebo "control" group. Results for other
treatments are listed separately - metformin,
fluvoxamine.
Authors include metformin patients in the control group,
allowing details of adjustments to affect results. Using standard treatment
vs. placebo analysis shows 61% lower hospitalization, or 75% lower for
patients with onset ≤5 days (not statistically significant with only 7 and 5
events). These results are not reported in the paper or the supplementary
appendix, readers need to request the data. Authors note that
"hospitalization is perhaps the most accurate and well-documented end
point".
There are many major issues as detailed below. We provide more
detailed analysis of this study due to widespread incorrect press. Submit Updates or
Corrections
| Author responses | |
| 17. Results sent to the US government1. Note most people live outside the US, and there was no action. | |
| No response for all other items | |
Ivermectin vs. placebo analysis - 61% lower hospitalization.
Authors include metformin patients in the control group, allowing details of adjustments to affect results. Using standard treatment (ivermectin only) vs. placebo analysis shows more favorable results for ivermectin, with 61% lower hospitalization, or 75% lower for patients with onset ≤5 days (not statistically significant with only 7 and 5 events). Authors note that "hospitalization is perhaps the most accurate and well-documented end point".
Severity mismatch for ivermectin treatment but not for any other medication or control.
The table shows the percentage of patients reporting severe dyspnea for each active treatment and respective control. We expect that patients reporting ER visits would be more likely to experience severe dyspnea. This is true for all cases except for ivermectin treatment, suggesting unlucky randomization for ivermectin treatment, or a potential data error. The percentages are with respect to the total number of patients reporting symptom data in each case.
| IVM active | IVM control | MF active | MF control | FLV active | FLV control | |
|---|---|---|---|---|---|---|
| ER | 0.0% | 9.1% | 13.3% | 10.0% | 10.0% | 14.3% |
| Non-ER | 6.1% | 6.9% | 8.0% | 7.8% | 6.4% | 8.4% |
ER results unreliable, not related to symptoms.
Authors detail why the main hypoxemia results are unreliable, however the ER results appear to be similarly uninformative. ER visits do not appear to be related to symptoms. The mean total COVID-19 symptom score for patients reporting an ER visit is 55 compared to 56 for patients reporting no ER visit (or hospitalization/death). Visualization of the ER patient symptoms raises the question of why most of them went to the ER. Of the 26 patients reporting an ER visit and symptom data, only one ever reported severe dyspnea, 5 more reported at most moderate dyspnea, 11 more reported at most mild dyspnea, and 9 reported no dyspnea at any time. ER patients were less likely to report severe or moderate dyspnea. The decision to go to the ER appears to be more of a personal preference rather than based on symptoms. Patients that signed up for the trial may be especially concerned about PASC for example, and seek help based on potential future problems rather than current symptoms.
| Maximum dyspnea severity | ER patients | Non-ER/hosp./death patients |
|---|---|---|
| Severe | 3.8% | 6.5% |
| Moderate | 19.2% | 22.2% |
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
| D1 | D14 | ||||||||||||
Mismatch with reported death and symptoms.
There was only one death for a patient that was treated very late (7 days). The patient was not hospitalized. The death is reported within 14 days, however the patient reported symptom data for all 14 days, showing substantial recovery several days prior, with only 2 of 14 symptoms remaining and reported as mild. Data suggests that the death was not due to COVID-19.
| D1 | D14 | ||||||||||||
Ivermectin vs. placebo symptoms consistent with efficacy.
Authors include metformin patients in the control group, allowing details of adjustments to affect results. Using standard treatment vs. placebo analysis gives the mean COVID-19 symptom scores below, matching expectation for an effective treatment with the low-risk fast recovering population (note that administration on an empty stomach is expected to delay the time when therapeutic effects may be reached).
Multiple outcomes missing, including time to recovery.
Multiple outcomes are missing, for example time to recovery (where ACTIV-6 showed superiority of ivermectin): "Time to meaningful recovery (symptoms or severity improved by one category and sustained for at least 36 hours)" (protocol page 91). Notably, the definition is less biased than the ACTIV-6 definition, including improvement by one category, making allowance for mild fatigue and cough, and requiring 36 hours sustained rather than 3 days. More notably, the result is not reported.
Hypoxemia results unreliable but prioritized.
Authors detail why the hypoxemia results are unreliable2 @28:30, however they are still prioritized in the presentation, and included in the abstract without mentioning that these results are unreliable.
Adverse events suggest authentic ivermectin not taken.
Adverse events were notably not reported in the paper, other than to
note none were serious. Partial information is contained in Table S2 and
Figure S5. Notably, there is no significant increase for ivermectin for
any of the expected side effects, in contrast to other trials, e.g.3. These results are unexpected if patients received and took
authentic ivermectin at the dosage indicated. Adverse events have been
reported to clinicaltrials.gov, which shows only one adverse avent
(neuropathy) for all 410 ivermectin patients, which does not match Table
S24.
Major event counts differ between paper and registry.
The main outcome event numbers are different between the paper and the
clinicaltrials.gov registry. Some differences are expected -
clinicaltrials shows events for each arm while the paper hides information
by using control groups with other treatments instead of placebo
comparisons. However, expected matches are different. For example, the
paper shows 8/652 hospitalization or death for metformin, while the
registry shows 18/652 for all treatment groups with metformin4. It
appears that authors attempted to submit the combined data that hides the
individual arm results (which show lower hospitalization for ivermectin
versus placebo) but their submission was not allowed and they subsequently
submitted false data. For discussion see5.
Baseline data differs between paper and registry.
The baseline data is different between the paper and the
clinicaltrials.gov registry. For example, for ivermectin the paper shows
19/410 Asian patients, while the registry shows 13/4104. For
metformin, the numbers are 25/663 and 18/663.
Control group includes metformin, adjustment protocol violation.
The "control" group includes patients receiving metformin, which is
known to be beneficial for COVID-196. Authors present adjusted
results however they do not appear to fully account for metformin
efficacy. For example, the adjusted result for ivermectin ER/hosp./death
is close to the unadjusted result, while a greater difference would be
expected based on the metformin efficacy reported (which is not expected
to be doubled in the metformin + ivermectin arm). The trial has 5
treatments arms, but is presented as if there was 3, which adds
complexity, makes the results subject to potential interactions between
treatments, and introduces the potential for investigator bias in
adjustments. Notably, the protocol specifies primary and secondary
adjustments (page 74), and the paper reports only one set of adjustments,
which matches neither the primary or secondary adjustments in the
protocol.
Primary outcome changes.
The primary outcome was changed around and after the end of recruitment7,8.
All 7 secondary outcomes deleted.
All 7 secondary outcomes were deleted in the
clinicaltrials.gov registry on April 18, 20238.
Metformin/fluvoxamine conclusions opposite of Together Trial, but matching earlier studies on each team.
The Together trial and COVID-OUT both tested metformin and fluvoxamine. Notably, they came to opposite conclusions. In Together, authors found efficacy for fluvoxamine, but the metformin results were so negative that the trial was terminated early. In COVID-OUT it was the opposite, authors (although not the journal editor) found efficacy for metformin, while the fluvoxamine results were so negative that the trial was terminated early9. Note that the Together authors include researchers that found fluvoxamine effective in earlier studies, while the COVID-OUT authors include researchers that found metformin effective in earlier studies.
Author claims results from 662 researchers should be censored for false information.
65 studies by 662 scientists report statistically significant positive results for ivermectin treatment of COVID-1910. One author claimed that a report of positive results is "disinformation" and distributed a request to report and censor the author11-13. While discussion is warranted for all studies, a call for censorship of results is extreme and raises questions. Author provides no basis for the results of the 662 scientists being wrong and warranting of censorship, and there is no indication that author has even read most of the studies. Author cherry-picked two of 106 studies, (COVID-OUT and ACTIV-614,15, both very high COI studies with an extensive list of issues and very delayed treatment) and claimed that "no benefit of ivermectin was observed"16. In addition to ignoring the 65 studies reporting statistically significant positive results, ACTIV-617 reported a posterior probability that ivermectin is effective of 99%, 98%, and 97% for mean time unwell, clinical progression @14 days, and clinical progression @7 days (even though none of the pre-specified primary outcomes were reported, and noting that these preprint results were changed without explanation), and COVID-OUT showed 61% lower hospitalization with ivermectin vs. placebo (not including metformin), although this was not reported.
Administration on an empty stomach.
Authors instructed patients to take ivermectin on an empty stomach, but
other treatments with food. Guzzo show that the plasma
concentration of ivermectin is much higher when administered with food
(geometric mean AUC 2.6 times higher). "Ivermectin or matching placebo
should be taken by mouth on an empty stomach with water. 1 hour before or
2 hours after a meal. All other agents should be taken by mouth at the end
of a balanced snack or small meal."
Results delayed 6 months (including life-saving metformin results).
Results were delayed for 6 months with no
explanation, with followup ending Feb 14, 2022. Results were not presented
until July 819,
and they were still not available to the public due to a news embargo for
over a month. Embargo and delay of clinical trial results during a
pandemic is not consistent with a goal of minimizing mortality and
morbidity. Notably authors report very positive results for metformin
(although journal editors changed the conclusion as below).
Subject to participant fraud.
The self-reported design and absence of professional medical examination opens this kind of remote trial to participant fraud, which may be significant due to extreme politicization in the study country. Participant fraud has been reported for two other remote trials with a shared author20,21, involving submission of fake surveys and repeated signups.
Fewer comorbidities for serious outcomes.
Patients experiencing serious outcomes are expected to be more likely to have comorbidities, however the opposite is seen.
| Outcome | Comorbidity prevalence |
|---|---|
| Non-ER/hosp./death | 53% |
| ER | 45% |
| Hospitalization | 12.5% |
| Death | 0% |
Control arm results very different between treatments.
Control arm results are very different between treatments, for example
considering hospitalization/death, this was 1.0% for ivermectin treatment
vs. 2.7% for metformin control, however it was 1.3% for the ivermectin
control. The metformin arm started earlier, however the difference in
outcomes is very large given that most patients are in the shared
period.
COVID-19 specific symptoms hidden in appendix.
Authors present results for all symptoms in Figure 2, and for COVID-19
symptoms in the appendix Figure S4. Notably, the COVID-19 specific results
are better for ivermectin and especially for fluvoxamine.
Authors claim placebo is not better than the treatments.
Authors state: Neither overall symptoms nor Covid-19–specific
symptoms were reduced faster with placebo than with any of the trial
drugs. This may be true, Figure S4 shows symptoms were reduced faster
with all treatments (with ivermectin and fluvoxamine showing greater
improvement than metformin), but the reverse claim is very unusual —
placebo is not expected to be better. Note that the graphs and data refer
to the control groups including other treatments, while the statement
refers to placebo only.
Incorrect claim that no treatment reduced severity.
Authors claim that "None of the trial drugs resulted in a lower severity of symptoms than identically matched placebo." The intended meaning — compared to the "control" groups used, since that is the data reported — is incorrect, multiple results show lower severity in the treatment groups in terms of the symptom scores and severity resulting in hospitalization. Individual results may not reach statistical significance, however ER/hosp./death does in the larger metformin group.
False conclusion.
Authors claim "None of the three medications that were evaluated
prevented the occurrence of hypoxemia, an emergency department visit,
hospitalization, or death associated with Covid-19." Taking the
literal wording, this is false, there were no deaths with fluvoxamine.
Taking the likely meaning (no treatment reduced incidence of these
events), this is false, reduced incidence is seen in several results
(mostly without statistical significance).
Trial outcomes modified.
Trial outcomes were changed on January 20, 202222,
and again on March 2, 202223.
Very high percentage of missing data.
There is a very high percentage of missing data. 25% of patients have zero symptom data reported for all 14 days in the data file. This does not match the paper which reports 20% of patients did not contribute symptom data (Figure 2).
Medication delivery varied significantly.
Medication delivery varied significantly over the trial. In this
presentation24, author indicates that
delivery was initially local, later via FedEx, was much slower in August,
there were delays due to team bandwidth issues, and they only realized
they could use FedEx same day delivery in September.
Treatment 3 days for ivermectin, 14 days for metformin and fluvoxamine.
Treatment was 14 days for metformin and fluvoxamine, but only 3
days for ivermectin.
SAP dated after trial.
The SAP is dated February 14, 2022, which authors note is one day
before unblinding. However, the protocol notes that the statisticians are
unblinded: "There is one unblinded statistician with two unblinded
supporting statisticians on the study team", and "All analyses will
be carried out by the un-blinded statisticians". The protocol also
notes that the SAP will be developed by unblinded statisticians in one
case, and blinded in a second case: "detailed statistical analysis plan
will be developed by the unblinded statisticians", and "statistical
analysis plan will be developed by the blinded statistician."
Test requirement and delivery prohibits early treatment.
The requirement for a positive test and delivery of medication
introduces substantial delay and largely excludes the possibility of early
treatment. The protocol requires verifiable results using a local
laboratory standard which excludes most home antigen tests (supplementary
data page 5). Note that the trial results do not generalize to real-world
usage, where clinicians recommend treatment immediately on symptoms.
Conclusion modified by journal.
Author statements indicate that the conclusion was modified by the journal25,26.
Symptom results contradictory.
Authors consider only metformin results to be positive (the journal
editor considers none to be positive), however the symptom results in
Figure S4 show the opposite: ivermectin and fluvoxamine show faster
improvement (without statistical significance), while no difference is
seen for metformin.
Adherence very low.
Adherence was very low, with 77% overall reporting 70+% adherence, and
85% for ivermectin reporting 70+% adherence. An author has claimed 85%
took all doses but that is contradicted by the 20% reported "Total
Interruption or Discontinuation" in Table S2. Numbers for 100% adherence
are not provided.
Inconsistent blinding statements.
Protocol page 12 states that "The research team statisticians will remain blinded", while the supplementary data page 40 states that "There is one unblinded statistician with two unblinded supporting statisticians on the study team".
Author indicates a best guess can be used for onset.
One author suggests that investigators can use a "best guess" if a patient gives a range for time of onset, which would allow a biased investigator to present an incorrect lower average time from onset27.
Ivermectin from source chosen has shown lower efficacy.
Authors chose to source ivermectin from Edenbridge, which ranked 7 out of 11 brands in In Vitro tests for antiparasitic efficacy28, requiring 5 days compared to 2 days for the best performing brand, and 3 days for 4 other brands.
Highest mean age for ivermectin, lowest for placebo.
All treatment groups show the same median age (46) in the paper,
however the clinicaltrials.gov registry shows the mean ages, and the mean
is notably higher in the ivermectin only group (48) versus all other
groups, suggesting a skew towards older patients specifically in the
ivermectin only group. The control median age for ivermectin is 45 in the
paper, while the placebo mean age in the registry is 42, while no other
group has a mean age below 46. There is a large difference between the
ivermectin and placebo mean ages (48 vs. 42), which is hidden in the paper
which shows median 46 vs. 45 ivermectin vs. control
Adherence subgroups analysed but not reported.
Authors indicate they performed subgroup analysis by adherence2 @18:30, however these results have
not been reported.
Maximum symptom duration not clear.
The procol excludes patients with >7 days of symptoms, i.e. patients 7
days from onset are included. The paper claims "less than 7 days"
in one instance and "within 7 days" in another. The presentation
reports "<7 days"2.
No discontinuation due to hospitalization for ivermectin.
Table S2 shows 9 placebo patients discontinued treatment due to hospitalization, compared to zero for ivermectin. While ivermectin patients only received 3 days treatment, they received placebo tablets for the remaining days. If this number is only counting discontinuation during the first three days, the result highlights that treatment was stopped before any patients were hospitalized. The protocol notes "Study drug will be stopped at the time of hospitalization for any reason".
Authors indicate up to 5 day delay in real-world usage.
Authors note up to 11 days treatment delay with a remote clinical trial
compared to up to 5 day for "real-world use"2 @43:00, where the 5 days derives
from testing and medical system delays. However, logical real-world use,
as used in many locations, is to have the treatment on hand to take
immediately.
This is the 41st of 53 COVID-19 RCTs for ivermectin, which collectively show efficacy with p=0.000000087.
This is the 90th of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments29.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers ivermectin, fluvoxamine, and metformin.
|
risk of death, 197.1% higher, RR 2.97, p = 1.00, treatment 1 of 408 (0.2%), control 0 of 396 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28.
|
|
risk of death/hospitalization, 26.7% lower, RR 0.73, p = 0.66, treatment 4 of 406 (1.0%), control 5 of 394 (1.3%), NNT 352, odds ratio converted to relative risk.
|
|
risk of progression, 36.8% higher, RR 1.37, p = 0.33, treatment 23 of 406 (5.7%), control 16 of 394 (4.1%), odds ratio converted to relative risk, combined ER, hospitalization, death.
|
|
risk of progression, 3.7% higher, RR 1.04, p = 0.78, treatment 105 of 407 (25.8%), control 96 of 391 (24.6%), odds ratio converted to relative risk, combined hypoxemia, ER, hospitalization, death, primary outcome.
|
|
risk of hospitalization, 60.8% lower, RR 0.39, p = 0.28, treatment 2 of 206 (1.0%), control 5 of 202 (2.5%), NNT 66, IVM vs. placebo.
|
|
risk of hospitalization, 74.6% lower, RR 0.25, p = 0.37, treatment 1 of 137 (0.7%), control 4 of 139 (2.9%), NNT 47, IVM vs. placebo, ≤5 days from onset.
|
|
risk of hospitalization, 70.1% lower, RR 0.30, p = 0.12, treatment 3 of 406 (0.7%), control 5 of 202 (2.5%), NNT 58, IVM and IVM+MF vs. placebo.
|
|
risk of hospitalization, 41.8% lower, RR 0.58, p = 0.50, treatment 3 of 406 (0.7%), control 5 of 394 (1.3%), NNT 189, IVM and IVM+MF vs. placebo and MF.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
3.
Lim et al., Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial, JAMA, doi:10.1001/jamainternmed.2022.0189.
7.
classic.clinicaltrials.gov, classic.clinicaltrials.gov/ct2/history/NCT04510194?A=15&B=16&C=merged#StudyPageTop.
8.
classic.clinicaltrials.gov (B), classic.clinicaltrials.gov/ct2/history/NCT04510194?A=18&B=19&C=merged#StudyPageTop.
14.
Bramante et al., Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, NEJM, doi:10.1056/NEJMoa2201662.
15.
Naggie et al., Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.18590.
18.
Guzzo et al., Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects, J. Clinical Pharmacology, doi:10.1177/009127002237994.
19.
rethinkingclinicaltrials.org, rethinkingclinicaltrials.org/event/grand-rounds-july-8-results-from-the-covid-out-trial-a-phase-3-trial-of-outpatient-treatment-for-covid-19-using-metformin-ivermectin-and-fluvoxamine/.
21.
Lindsell et al., ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19, Journal of Clinical and Translational Science, doi:10.1017/cts.2023.644.
22.
clinicaltrials.gov (B), clinicaltrials.gov/ct2/history/NCT04510194?A=15&B=16&C=merged#StudyPageTop.
23.
clinicaltrials.gov (C), clinicaltrials.gov/ct2/history/NCT04510194?A=16&B=17&C=merged#StudyPageTop.
Bramante et al., 18 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 37 authors, average treatment delay 4.6 days, dosage 430μg/kg days 1-3, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04510194 (history) (COVID-OUT).
Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2201662
BACKGROUND Early treatment to prevent severe coronavirus disease 2019 (Covid-19) is an important component of the comprehensive response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic.
METHODS In this phase 3, double-blind, randomized, placebo-controlled trial, we used a 2-by-3 factorial design to test the effectiveness of three repurposed drugs -metformin, ivermectin, and fluvoxamine -in preventing serious SARS-CoV-2 infection in nonhospitalized adults who had been enrolled within 3 days after a confirmed diagnosis of infection and less than 7 days after the onset of symptoms. The patients were between the ages of 30 and 85 years, and all had either overweight or obesity. The primary composite end point was hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death. All analyses used controls who had undergone concurrent randomization and were adjusted for SARS-CoV-2 vaccination and receipt of other trial medications.
RESULTS A total of 1431 patients underwent randomization; of these patients, 1323 were included in the primary analysis. The median age of the patients was 46 years; 56% were female (6% of whom were pregnant), and 52% had been vaccinated. The adjusted odds ratio for a primary event was 0.84 (95% confidence interval [CI], 0.66 to 1.09; P = 0.19) with metformin, 1.05 (95% CI, 0.76 to 1.45; P = 0.78) with ivermectin, and 0.94 (95% CI, 0.66 to 1.36; P = 0.75) with fluvoxamine. In prespecified secondary analyses, the adjusted odds ratio for emergency department visit, hospitalization, or death was 0.58 (95% CI, 0.35 to 0.94) with metformin, 1.39 (95% CI, 0.72 to 2.69) with ivermectin, and 1.17 (95% CI, 0.57 to 2.40) with fluvoxamine. The adjusted odds ratio for hospitalization or death was 0.47 (95% CI, 0.20 to 1.11) with metformin, 0.73 (95% CI, 0.19 to 2.77) with ivermectin, and 1.11 (95% CI, 0.33 to 3.76) with fluvoxamine.
CONCLUSIONS None of the three medications that were evaluated prevented the occurrence of hypoxemia, an emergency department visit, hospitalization, or death associated with Covid-19. (Funded by the Parsemus Foundation and others; COVID-OUT ClinicalTrials .gov number, NCT04510194.
Appendix The authors' full names and academic degrees are as follows: Carolyn T. Bramante
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Blitzer, Ham, Colby, Skondra, Association of metformin use with age-related macular degeneration: a casecontrol study, JAMA Ophthalmol
Bolo, Hodé, Nédélec, Lainé, Wagner et al., Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy, Neuropsychopharmacology
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19
Bramante, Buse, Tamaritz, Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity, J Med Virol
Bramante, Ingraham, Murray, Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis, Lancet Healthy Longev
Bramante, Proper, Boulware, Vaccination against SARS-CoV-2 is associated with a lower viral load and Metformin, Ivermectin, and Fluvoxamine for Covid-19 likelihood of systemic symptoms, Open Forum Infect Dis
Campo, García-Valdecasas, Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy, PLoS One
Cariou, Hadjadj, Wargny, Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study, Diabetologia
Castle, Dock, Hemmat, Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets, doi:10.1101/2020.05.22.111237v2
Cheng, He, Jung, Lu, Gao, Suppression of Kaposi's sarcoma-associated herpesvirus infection and replication by 5′-AMP-activated protein kinase, J Virol
Connors, Brooks, Sciurba, Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial, JAMA
Crouse, Grimes, Li, Might, Ovalle et al., Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes, Front Endocrinol
Dodds, Doyle, Reiersen, Brown, Rayner, Fluvoxamine for the treatment of COVID-19, Lancet Glob Health
Facente, Reiersen, Lenze, Boulware, Klausner, Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence, Drugs
Farfan-Morales, Cordero-Rivera, Anti-flavivirus properties of lipid-lowering drugs, Front Physiol
Ferdinands, Rao, Dixon, Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance -VISION Network, 10 states, August 2021, MMWR Morb Mortal Wkly Rep
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Hales, Carroll, Fryar, Ogden, Prevalence of obesity and severe obesity among adults: United States, 2017-2018, NCHS Data Brief
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for highrisk, nonhospitalized adults with Covid-19
Karam, Morris, Bramante, mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses, J Med Virol
Keehner, Horton, Binkin, Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce, N Engl J Med
Lee, Vigod, Bortolussi-Courval, Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis, JAMA Netw Open
Lenze, Mattar, Zorumski, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Li, Yang, Yan, Sun, Zeng et al., Metformin in patients with COVID-19: a systematic review and meta-analysis, Front Med
Luo, Qiu, Liu, Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis, Am J Trop Med Hyg
López-Medina, López, Hurtado, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA
Marzolini, Kuritzkes, Marra, Prescribing nirmatrelvir-ritonavir: how to recognize and manage drug-drug interactions, Ann Intern Med
Medical, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA
Meng, Db, Performing likelihood ratio tests with multiply-imputed data sets, Biometrika
Nakashima, Takeuchi, Chihara, Hotta, Sada, Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways, Microbiol Immunol
Newberry, Williams, Stauffer, Boulware, Hendel-Paterson et al., Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis, Chest
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Oldenburg, Pinsky, Brogdon, Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial, JAMA
Popp, Stegemann, Metzendorf, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev
Portmann-Baracco, Alberti, Accinelli, Antiviral and anti-inflammatory properties of ivermectin and its potential use in COVID-19, Arch Bronconeumol (Engl Ed)
Postler, Peng, Bhatt, Ghosh, Metformin selectively dampens the acute inflammatory response through an AMPK-dependent mechanism, Sci Rep
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health
Reis, Santos, Silva, Silva, Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial, Lancet Reg Health Am
Schaller, Sharma, Dupee, Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses, JCI Insight
Seftel, Boulware, Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19, Open Forum Infect Dis
Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Intern Med
Stauffer, Alpern, Walker, COVID-19 and dexamethasone: a potential strategy to avoid steroid-related strongyloides hyperinfection, JAMA
Thompson, Natarajan, Irving, Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance -VISION Network, 10 states, August 2021, MMWR Morb Mortal Wkly Rep
Timmins, Donahue, Meeker, Marathe, Steady-state pharmacokinetics of a novel extended-release metformin formulation, Clin Pharmacokinet
Xian, Liu, Nilsson, Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation, Immunity
Xin, Wei, Ji, Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release, Sci Rep
Yu, Sun, Zhao, Kang, Yan, The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance, Int J Infect Dis
DOI record:
{
"DOI": "10.1056/nejmoa2201662",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2201662",
"alternative-id": [
"10.1056/NEJMoa2201662"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5858-2080",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Huling",
"given": "Jared D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Tignanelli",
"given": "Christopher J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9723-3876",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Buse",
"given": "John B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Liebovitz",
"given": "David M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Nicklas",
"given": "Jacinda M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Cohen",
"given": "Kenneth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6358-4670",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Puskarich",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Belani",
"given": "Hrishikesh K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6037-4077",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Proper",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Siegel",
"given": "Lianne K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Klatt",
"given": "Nichole R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7731-2799",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Odde",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Luke",
"given": "Darlette G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Anderson",
"given": "Blake",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2781-3824",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Karger",
"given": "Amy B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0292-0594",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Ingraham",
"given": "Nicholas E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6723-0423",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Hartman",
"given": "Katrina M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Rao",
"given": "Via",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Hagen",
"given": "Aubrey A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Patel",
"given": "Barkha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Fenno",
"given": "Sarah L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Avula",
"given": "Nandini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Reddy",
"given": "Neha V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Erickson",
"given": "Spencer M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Lindberg",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Fricton",
"given": "Regina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Lee",
"given": "Samuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6884-7748",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Zaman",
"given": "Adnin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Saveraid",
"given": "Hanna G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Tordsen",
"given": "Walker J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Pullen",
"given": "Matthew F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Biros",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Sherwood",
"given": "Nancy E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Thompson",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Departments of Medicine (C.T.B., N.E.I., K.M.H., A.A.H., B.P., S.L.F., N.A., N.V.R., S.M.E., H.G.S., M.F.P., D.R.B.) and Surgery (C.J.T., N.R.K.), Emergency Medicine (M.A.P., M.B.), and Laboratory Medicine and Pathology (A.B.K.), Medical School, the Divisions of Biostatistics (J.D.H., J.L.P., L.K.S., V.R., S. Lindberg, T.A.M.) and Epidemiology and Community Health (N.E.S.), School of Public Health, and the Department of Biomedical Engineering (D.J.O.), University of Minnesota, the..."
}
],
"family": "Murray",
"given": "Thomas A.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
17
]
],
"date-time": "2022-08-17T21:07:21Z",
"timestamp": 1660770441000
},
"deposited": {
"date-parts": [
[
2024,
3,
26
]
],
"date-time": "2024-03-26T22:20:11Z",
"timestamp": 1711491611000
},
"funder": [
{
"name": "UnitedHealth Group Foundation"
},
{
"DOI": "10.13039/100016127",
"doi-asserted-by": "publisher",
"name": "Fast Grants"
},
{
"DOI": "10.13039/100006108",
"award": [
"KL2TR002492",
"UL1TR002494"
],
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
},
{
"DOI": "10.13039/100000062",
"award": [
"DK124654-01A1"
],
"doi-asserted-by": "publisher",
"name": "National Institute of Diabetes and Digestive and Kidney Diseases"
},
{
"DOI": "10.13039/100016608",
"doi-asserted-by": "publisher",
"name": "Rainwater Charitable Foundation"
},
{
"name": "The Parsemus Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T20:31:04Z",
"timestamp": 1712608264384
},
"is-referenced-by-count": 139,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
8,
18
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2022,
8,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
18
]
],
"date-time": "2022-08-18T00:00:00Z",
"timestamp": 1660780800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2201662",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "599-610",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
8,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
18
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMc2112981",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_2_2"
},
{
"DOI": "10.15585/mmwr.mm7107e2",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.15585/mmwr.mm7104e3",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1056/NEJMoa2109682",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.7326/M22-0281",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1101/2020.05.22.111237",
"doi-asserted-by": "crossref",
"key": "e_1_3_4_9_2",
"unstructured": "Castle BT Dock C Hemmat M et al. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. June 16 2020 (https://www.biorxiv.org/content/10.1101/2020.05.22.111237v2). preprint."
},
{
"DOI": "10.1002/jmv.26728",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2"
},
{
"DOI": "10.3389/fphys.2021.749770",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.1128/JVI.00624-16",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1371/journal.pone.0191805",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1111/j.1348-0421.2011.00382.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1172/jci.insight.148003",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1038/s41598-021-97441-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1038/srep36222",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1016/j.immuni.2021.05.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1002/jmv.26873",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.4269/ajtmh.20-0375",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1007/s00125-020-05180-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.3389/fmed.2021.704666",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
},
{
"DOI": "10.3389/fendo.2020.600439",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_2"
},
{
"DOI": "10.1016/S2666-7568(20)30033-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_25_2"
},
{
"DOI": "10.1016/j.arbr.2020.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_2"
},
{
"article-title": "Ivermectin for preventing and treating COVID-19.",
"author": "Popp M",
"first-page": "CD015017",
"issue": "7",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_3_4_27_2",
"unstructured": "Popp M, Stegemann M, Metzendorf M-I, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021;7(7):CD015017-CD015017.34318930",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_28_2"
},
{
"DOI": "10.1007/s40265-021-01636-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_2"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_30_2"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_31_2"
},
{
"DOI": "10.1001/jama.2020.22760",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_32_2"
},
{
"DOI": "10.1093/ofid/ofab050",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_33_2"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_34_2"
},
{
"key": "e_1_3_4_35_2",
"unstructured": "Food and Drug Administration. Pulse oximeter accuracy and limitations: FDA safety communication. February 19 2021 (https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication)."
},
{
"key": "e_1_3_4_36_2",
"unstructured": "Food and Drug Administration. Multiple endpoints in clinical trials: guidance for industry. January 2017 (https://www.fda.gov/media/102657/download)."
},
{
"key": "e_1_3_4_37_2",
"unstructured": "Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. September 2020 (https://www.fda.gov/media/142143/download)."
},
{
"key": "e_1_3_4_38_2",
"unstructured": "Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. FDA news release. February 11 2022 (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-monoclonal-antibody-treatment-covid-19-retains)."
},
{
"DOI": "10.1093/ofid/ofac066",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_39_2"
},
{
"DOI": "10.1001/jama.2013.281053",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_40_2"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_41_2"
},
{
"DOI": "10.1001/jama.2021.17272",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_42_2"
},
{
"DOI": "10.1093/biomet/79.1.103",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_43_2"
},
{
"article-title": "Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial.",
"author": "Reis G",
"first-page": "100142",
"journal-title": "Lancet Reg Health Am",
"key": "e_1_3_4_44_2",
"unstructured": "Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022;6:100142-100142.34927127",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1001/jamaophthalmol.2020.6331",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_45_2"
},
{
"DOI": "10.2165/00003088-200544070-00004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_46_2"
},
{
"DOI": "10.1016/S0893-133X(00)00116-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_47_2"
},
{
"DOI": "10.1016/S2214-109X(22)00006-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_48_2"
},
{
"DOI": "10.1378/chest.128.5.3681",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_49_2"
},
{
"DOI": "10.1001/jama.2020.13170",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_50_2"
},
{
"DOI": "10.1016/j.ijid.2012.02.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_51_2"
},
{
"article-title": "Prevalence of obesity and severe obesity among adults: United States, 2017-2018.",
"author": "Hales CM",
"first-page": "1",
"journal-title": "NCHS Data Brief",
"key": "e_1_3_4_52_2",
"unstructured": "Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;360:1-8.32487284",
"volume": "360",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11517",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_53_2"
}
],
"reference-count": 52,
"references-count": 52,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.742308258.793595134",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2201662"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19",
"type": "journal-article",
"volume": "387"
}
covidout