ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19
et al., Journal of Clinical and Translational Science, doi:10.1017/cts.2023.644, NCT04885530, Oct 2023
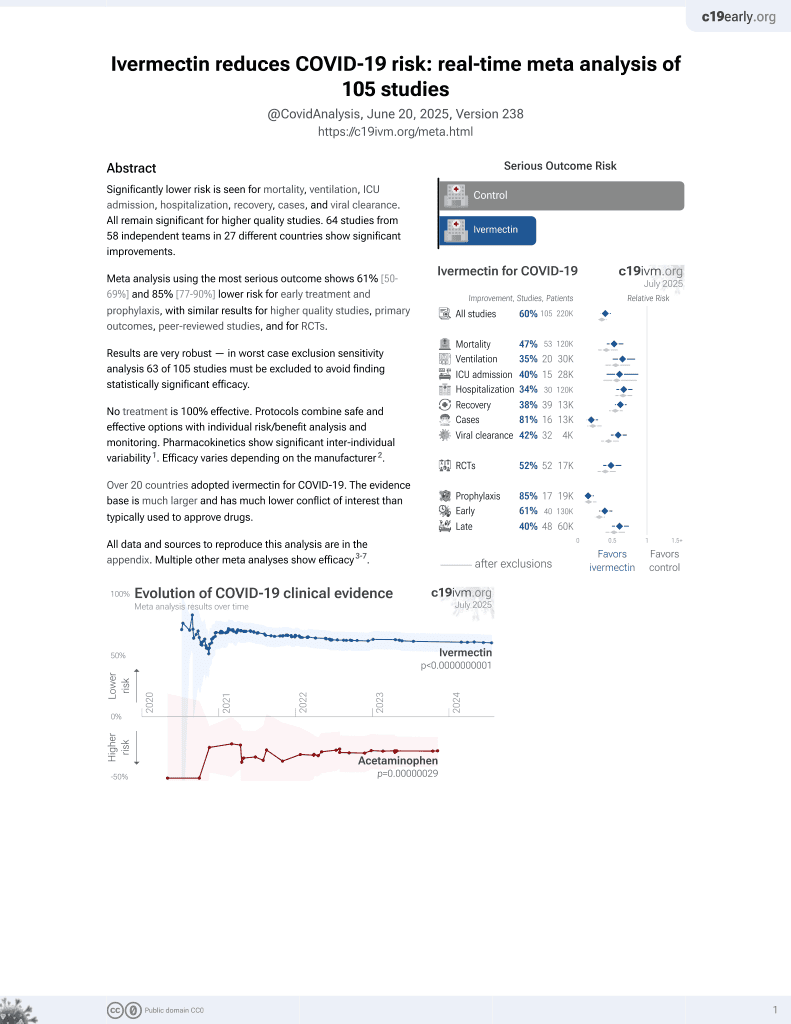
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Discussion of the operation of ACTIV-61 revealing participant fraud - authors identified participants that signed up repeatedly, and participants that withdrew when not randomized to their preferred arm. Authors indicate that they tried to prevent repeat signups but provide no details on the algorithms or the evaluation thereof. It is possible that they only caught a small fraction of the fraud, and possible that improvements to detection were added only later in the trial.
It is likely that individuals were gaming the system related to politicized treatments with extreme financial implications. This information was not disclosed previously.
Authors note that delivery was increasingly made to centralized pickup locations and not directly to the participant. Authors indicate they use shipping logs and participant notification of drug receipt. This is unclear because if delivery was based on participant confirmation there would be no need to determine delivery based on shipping logs. This suggests that in at least some cases, delivery time may not have accounted for the time for participant pickup. Therefore the actual treatment delay may be even longer than reported.
Lindsell et al., 31 Oct 2023, USA, peer-reviewed, 1 author, trial NCT04885530 (history).
Contact: chris.lindsell@duke.edu.
ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19
Journal of Clinical and Translational Science, doi:10.1017/cts.2023.644
Despite the availability of vaccinations, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to cause Coronavirus Disease 2019 (COVID-19) infection with a spectrum of disease in the acute setting. Transmission, infection, and severe disease remain common. There is a critical need to establish treatment regimens in the ambulatory setting that can reduce symptom burden and potentially prevent progression to severe disease and death. Many existing medicines previously approved for other uses may have benefit but remain unproven in informative clinical trials. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 is a decentralized, placebo-controlled, double-blind, randomized, platform trial that has now enrolled more than 7500 participants and has reported on the effectiveness of ivermectin at two doses, fluticasone, and fluvoxamine for helping people with COVID-19. With additional repurposed therapies added to the platform, ACTIV-6 continues to enroll symptomatic outpatients aged ≥ 30 years with a confirmed positive PCR or antigen test for SARS-CoV-2. Potential participants are screened and enrolled online, through a call center, or facilitated by local study sites. Participants consent electronically and are randomized to placebo or to one of the open study drugs for which they are eligible at the time of enrollment. A shared, contemporary placebo approach is used. Participants receive study drug in the mail and remain on study for up to 180 days. While enrolled, electronic patient-reported outcome assessments are used to monitor symptoms, healthcare utilization, and mortality. The primary endpoint is time to recovery or a composite of hospitalization and mortality within 28 days. Symptoms, acute healthcare utilization, and the Patient-Reported Outcomes Measurement Information System-29 are collected for up to 180 days. Using a decentralized trial approach allowed the ACTIV-6 platform to increase both reach and rate of enrollment. The decentralized approach did not simplify regulatory oversight, and we found unanticipated challenges in patient behavior and the study drug delivery process. Despite challenges, ACTIV-6 has enrolled thousands of participants from across the USA and continues to test the effectiveness of repurposed medicines for treating COVID-19. Our lessons learned contribute to the emerging understanding of how to optimize decentralized trials.
Competing interests. Dr Lindsell reported receiving grants to the institution from the National Center for Advancing Translational Sciences (NCATS) to the institution during the conduct of the study and grants to the institution from NIH and Department of Defense and research funds to the institution from the CDC, bioMerieux, AstraZeneca, AbbVie, Entegrion Inc., and Endpoint Health outside the submitted work; having a patent for risk stratification in sepsis and septic shock issued to Cincinnati Children's Hospital Medical Center; and having stock options in Bioscape Digital unrelated to the current work. Dr Naggie reported receiving grants from the National Institutes of Health (NIH) during the conduct of the study and receiving grants from Gilead Sciences and AbbVie; receiving personal fees from Pardes Biosciences and Silverback Therapeutics for consulting; serving as a scientific advisor for and having stock options in Vir Biotechnology; receiving personal fees from and serving on a data and safety monitoring board for Personal Health Insights; and serving on an event adjudication committee for Bristol Myers Squibb/PRA Health Sciences outside the submitted work. Dr Hernandez reported receiving grants from AstraZeneca, Merck, and Pfizer outside the submitted work. No other disclosures were reported. Dr Stewart reported receiving grants from Duke University as a subaward for ACTIV-6 from NIH during the conduct of the study and grants from NIH supported by grants from NCATS..
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bernal, Da, Gomes, Musungaie, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Buchman, Draghia-Akli, Adam, Accelerating coronavirus disease 2019 therapeutic interventions and vaccines-Selecting compounds for clinical evaluation in coronavirus disease 2019 clinical trials, Crit Care Med, doi:10.1097/CCM.0000000000005295
Cella, Riley, Stone, The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008, J Clin Epidemiol, doi:10.1016/j.jclinepi.2010.04.011
Collins, Stoffels, Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times, JAMA, doi:10.1001/jama.2020.8920
Hamm, Arnold, Denson, The ACTIV-6 stakeholder advisory committee: a model for virtual engagement in decentralized clinical trials, J Clin Transl Sci
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Johnson, COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence -25 U.S. Jurisdictions, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7104e2
Kern, Schöning, Chaccour, Hammann, Modeling of SARS-coV-2 treatment effects for informed drug repurposing, Front Pharmacol
Krolewiecki, Lifschitz, Moragas, Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100959
Lavange, Adam, Currier, Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics, Ann Intern Med, doi:10.7326/M21-1269
Mccarthy, Naggie, Boulware, Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.24100
Munipalli, Seim, Dawson, Knight, Dabrh, Post-acute sequelae of COVID-19 (PASC): a meta-narrative review of pathophysiology, prevalence, and management, SN Compr Clin Med, doi:10.1007/s42399-022-01167-4
Naggie, Boulware, Lindsell, Effect of higher-dose ivermectin for 6 Days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2023.1650
Naggie, Boulware, Lindsell, Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.18590
Navarro, Camprubí, Requena-Méndez, Safety of high-dose ivermectin: a systematic review and meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkz524
DOI record:
{
"DOI": "10.1017/cts.2023.644",
"ISSN": [
"2059-8661"
],
"URL": "http://dx.doi.org/10.1017/cts.2023.644",
"abstract": "<jats:title>Abstract</jats:title>\n\t <jats:p>Despite the availability of vaccinations, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to cause Coronavirus Disease 2019 (COVID-19) infection with a spectrum of disease in the acute setting. Transmission, infection, and severe disease remain common. There is a critical need to establish treatment regimens in the ambulatory setting that can reduce symptom burden and potentially prevent progression to severe disease and death. Many existing medicines previously approved for other uses may have benefit but remain unproven in informative clinical trials.</jats:p>\n\t <jats:p>Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 is a decentralized, placebo-controlled, double-blind, randomized, platform trial that has now enrolled more than 7500 participants and has reported on the effectiveness of ivermectin at two doses, fluticasone, and fluvoxamine for helping people with COVID-19. With additional repurposed therapies added to the platform, ACTIV-6 continues to enroll symptomatic outpatients aged ≥ 30 years with a confirmed positive PCR or antigen test for SARS-CoV-2. Potential participants are screened and enrolled online, through a call center, or facilitated by local study sites. Participants consent electronically and are randomized to placebo or to one of the open study drugs for which they are eligible at the time of enrollment. A shared, contemporary placebo approach is used. Participants receive study drug in the mail and remain on study for up to 180 days. While enrolled, electronic patient-reported outcome assessments are used to monitor symptoms, healthcare utilization, and mortality. The primary endpoint is time to recovery or a composite of hospitalization and mortality within 28 days. Symptoms, acute healthcare utilization, and the Patient-Reported Outcomes Measurement Information System-29 are collected for up to 180 days.</jats:p>\n\t <jats:p>Using a decentralized trial approach allowed the ACTIV-6 platform to increase both reach and rate of enrollment. The decentralized approach did not simplify regulatory oversight, and we found unanticipated challenges in patient behavior and the study drug delivery process. Despite challenges, ACTIV-6 has enrolled thousands of participants from across the USA and continues to test the effectiveness of repurposed medicines for treating COVID-19. Our lessons learned contribute to the emerging understanding of how to optimize decentralized trials.</jats:p>",
"alternative-id": [
"S2059866123006441"
],
"article-number": "e221",
"author": [
{
"affiliation": [],
"name": "The Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group",
"sequence": "first"
}
],
"container-title": "Journal of Clinical and Translational Science",
"container-title-short": "J. Clin. Trans. Sci.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T11:39:11Z",
"timestamp": 1698752351000
},
"deposited": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T11:39:17Z",
"timestamp": 1698752357000
},
"indexed": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T07:17:33Z",
"timestamp": 1698823053510
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 303,
"start": {
"date-parts": [
[
2023,
10,
31
]
],
"date-time": "2023-10-31T00:00:00Z",
"timestamp": 1698710400000
}
}
],
"link": [
{
"URL": "https://www.cambridge.org/core/services/aop-cambridge-core/content/view/S2059866123006441",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "56",
"original-title": [],
"prefix": "10.1017",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
31
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Cambridge University Press (CUP)",
"reference": [
{
"DOI": "10.1001/jama.2020.8920",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref7"
},
{
"DOI": "10.1001/jama.2023.1650",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref14"
},
{
"DOI": "10.15585/mmwr.mm7104e2",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref22"
},
{
"key": "S2059866123006441_ref15",
"unstructured": "15. U.S. Food and Drug Administration. COVID-19: Developing Drugs and Biological Products for Treatment or Prevention. Published June 23, 2021. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention). Accessed April 27, 2022."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref3"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref4"
},
{
"DOI": "10.3389/fphar.2021.625678",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref18"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref19"
},
{
"DOI": "10.1007/s42399-022-01167-4",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref23"
},
{
"key": "S2059866123006441_ref1",
"unstructured": "1. Center for Systems Science and Engineering at Johns Hopkins University. Coronavirus COVID-19 (2019-nCoV). (https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6). Accessed May 11, 2023."
},
{
"DOI": "10.1097/CCM.0000000000005295",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref9"
},
{
"key": "S2059866123006441_ref6",
"unstructured": "6. National Institutes of Health. Immunomodulators Summary Recommendations. COVID-19 Treatment Guidelines. (https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/summary-recommendations/). Accessed April 15, 2022."
},
{
"DOI": "10.1101/2022.07.12.22277548",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref12"
},
{
"key": "S2059866123006441_ref16",
"unstructured": "16. World Health Organization. COVID-19 Therapeutic Trial Synopsis. (https://cdn.who.int/media/docs/default-source/blue-print/covid-19-therapeutic-trial-synopsis.pdf?sfvrsn=44b83344_1&download=true). Accessed February 18, 2020."
},
{
"key": "S2059866123006441_ref5",
"unstructured": "5. U.S. Food and Drug Administration. Coronavirus (COVID-19) | Drugs. Published March 31, 2022. (https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs). Accessed April 15, 2022."
},
{
"DOI": "10.1016/j.jclinepi.2010.04.011",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref17"
},
{
"DOI": "10.1001/jama.2022.24100",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref13"
},
{
"DOI": "10.7326/M21-1269",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref8"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref2"
},
{
"DOI": "10.1001/jama.2022.18590",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref11"
},
{
"key": "S2059866123006441_ref21",
"unstructured": "21. Centers for Disease Control and Prevention. COVID Data Tracker. Published March 28, 2020. (https://covid.cdc.gov/covid-data-tracker). Accessed July 27, 2021."
},
{
"DOI": "10.1093/jac/dkz524",
"doi-asserted-by": "publisher",
"key": "S2059866123006441_ref20"
},
{
"article-title": "The ACTIV-6 stakeholder advisory committee: a model for virtual engagement in decentralized clinical trials",
"author": "Hamm",
"journal-title": "J Clin Transl Sci",
"key": "S2059866123006441_ref10"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cambridge.org/core/product/identifier/S2059866123006441/type/journal_article"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "ACTIV-6: Operationalizing a decentralized, outpatient randomized platform trial to evaluate efficacy of repurposed medicines for COVID-19",
"type": "journal-article",
"volume": "7"
}