A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management
et al., Annals of Medicine & Surgery, doi:10.1016/j.amsu.2021.102779, NCT04753619, Sep 2021
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
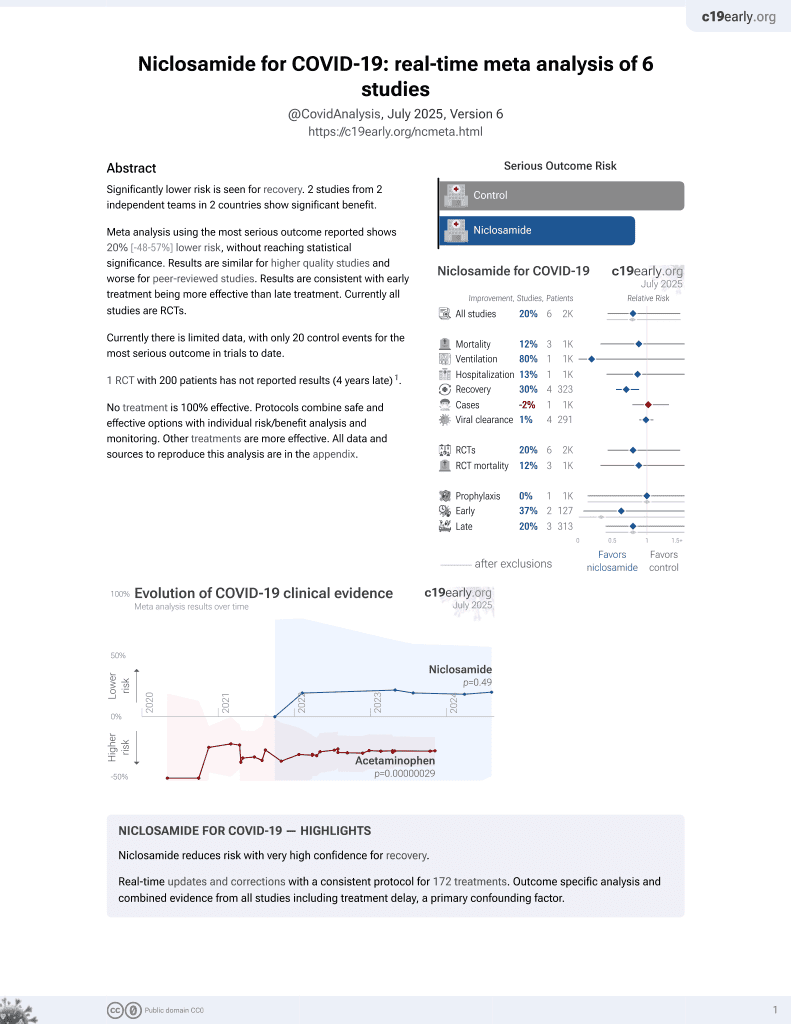
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT with 75 COVID-19 patients showing significantly faster recovery but no change in mortality with niclosamide.
The treatment group had more patients aged 60+ and more patients treated over a week after symptom onset.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 3 of 75 (4.0%), control 3 of 75 (4.0%).
|
|
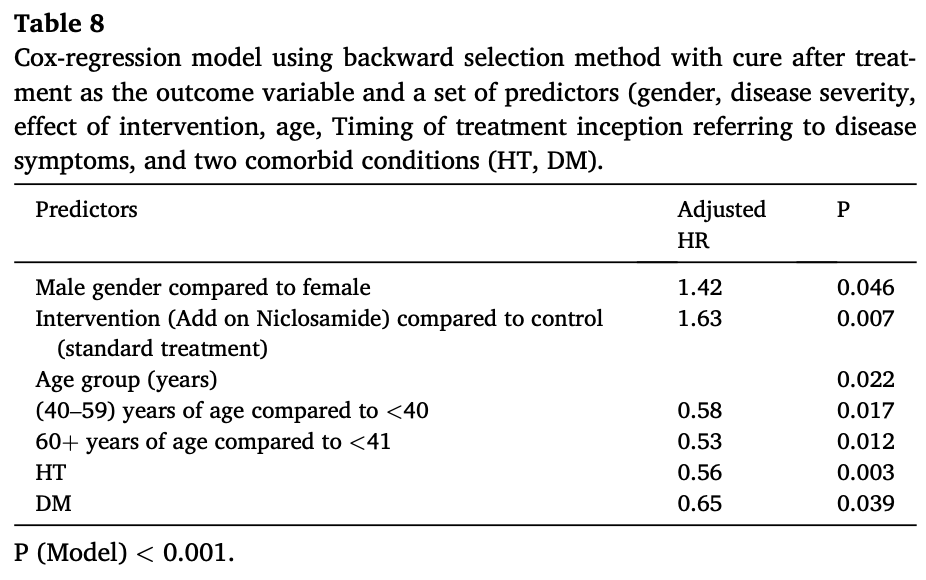
risk of no recovery, 38.7% lower, HR 0.61, p = 0.007, treatment 75, control 75, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Abdulamir et al., 30 Sep 2021, Randomized Controlled Trial, Iraq, peer-reviewed, 7 authors, trial NCT04753619 (history).
Contact: faiqig@gmail.com.
A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management
Annals of Medicine & Surgery, doi:10.1016/j.amsu.2021.102779
Background: COVID-19 pandemic has ignited the urge for repurposing old drugs as candidate antiviral medicines to treat novel challenges of viral infections. Niclosamide (NCS) is an anti-parasitic drug of known antiviral potential. Therefore, this study attempts to investigate the antiviral effect and safety of NCS on SARS-CoV-2 caused COVID-19 patients. Methods: Randomized controlled open label clinical trial encompassed 75 COVID-19 patients treated with standard of care plus NCS were included as experimental group and 75 COVID-19 patients treated with only standard of care therapy as control group. Survival rate, time to recovery, and side effects were the main endpoints for the assessment of the therapeutic effect and safety of NCS. Results: No significant difference between the two study groups in the incidence of death Vs recovery within 30 days of follow up(p = 1).Median survival time to cure in the NCS addon group was significantly less than controls (5 Vs 7days, Log rank p = 0.005).All the recoveries took place within 20 days in the NCS add on group, which is 10 days shorter than that in the controls (30 days), NCS add on treatment increased the risk of cure by 60% per day compared to control group (adjusted HR = 1.6,p = 0,007) after adjusting for the count of comorbidities. Additionally, two or more comorbidities reduced the risk of cure to 33% (p < 0.001).Male gender increased the risk of cure by 42% (p = 0.046). Older age group decreased the risk of recovery per day to 0.58 and 0.53 for 50-59 and 60+ years of age. Hyypertension (HT) and diabetes mellitus (DM) significantly reduced the risk of being cured per day to 0.56 (p = 0.003)and 0.65 (p = 0.039) respectively. No significant signals of safety in NCS add on therapy compared to control group. Conclusion: adding NCS to the standards of care measures increased the risk of the cure and had shorter time to stay in the hospital compared with controls., male gender increased the risk of cure, while older patients>40 years, HT, and DM decreased the risk of cure. Also, NCS add on therapy was relatively safe; hence, NCS is of clinical benefit for freeing hospital beds for more patients in pandemic crisis.
Author contribution All authors(contributed in concept or design of the study. NS, not significant.
Consent All patients signed written informed consent for participation in the study.
Registration of research Research registry UIN: researchregistry7040. At the website: www.re searchregistry.com/browse-the-registry#home/?view_2_search = researchregistry7040. &view_2_page = 1.
Guarantor Faiq I. Gorial.
Provenance and peer review Not commissioned, externally peer-reviewed.
Declaration of competing interest None.
References
Andrews, Thyssen, Lorke, The biology and toxicology of molluscicides, Bayluscide, Pharmacol Ther
Cabrita, Benedetto, Schreiber, Kunzelmann, Niclosamide repurposed for the treatment of inflammatory airway disease, JCI Insight, doi:10.1172/jci.insight.128414
Gassen, Niemeyer, Muth, SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nat. Commun, doi:10.1038/s41467-019-13659-4
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105949
Heymann, Hodgson, Sall, Zika virus and microcephaly: why is this situation a PHEIC?, Lancet, doi:10.1016/S0140-6736(16)00320-2
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog, doi:10.1371/journal.ppat.1002976
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog, doi:10.1371/journal.ppat.1002976
Jurgeit, Moese, Roulin, An RNA replication-center assay for high content image-based quantifications of human rhinovirus and coxsackievirus infections, Virol. J, doi:10.1186/1743-422X-7-264
Ko, Chang, Byun, Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19, Viruses, doi:10.3390/v13040651
Laujwy, Rosin, Agha, The CONSORT (CONsolidated standards of reporting trials) 2010 guideline, Int. J. Surg
Lin, Lu, Cao, Li, Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia, Emerg. Microb. Infect, doi:10.1080/22221751.2020.1746199
Lou, Sun, Rao, Current progress in antiviral strategies, Trends Pharmacol. Sci
Miner, Labitzke, Liu, Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways, Front. Pharmacol, doi:10.3389/fphar.2019.00051
Multicenter, Adaptive, Randomized Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Adults, ClinicalTrials
Pan, Ding, Wang, Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells, Chin. J. Canc, doi:10.5732/cjc.011.10290
Pindiprolu, Pindiprolu, CD133 receptor mediated delivery of STAT3 inhibitor for simultaneous elimination of cancer cells and cancer stem cells in oral squamous cell carcinoma, Med. Hypotheses, doi:10.1016/j.mehy.2019.109241
Rajamuthiah, Fuchs, Conery, Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus, PloS One, doi:10.1371/journal.pone.0124595
Wang, Lu, Lin, Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission, Antivir. Res, doi:10.1016/j.antiviral.2016.10.003
Wu, Jan, Chen, Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob. Agents Chemother, doi:10.1128/AAC.48.7.2693-2696.2004
Wu, Jan, Chen, Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob. Agents Chemother, doi:10.1128/AAC.48.7.2693-2696.2004
Xu, Shi, Li, Zhou, Broad spectrum antiviral agent niclosamide and its therapeutic potential, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00052
Xu, Shi, Li, Zhou, Broad spectrum antiviral agent niclosamide and its therapeutic potential, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00052
Yu, Du, Ojcius, Pan, Jiang, Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China, Microb. Infect, doi:10.1016/j.micinf.2020.01.003
Zhang, Liu, Potential interventions for novel coronavirus in China: a systematic review, J. Med. Virol, doi:10.1002/jmv.25707
DOI record:
{
"DOI": "10.1016/j.amsu.2021.102779",
"ISSN": [
"2049-0801"
],
"URL": "http://dx.doi.org/10.1016/j.amsu.2021.102779",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Annals of Medicine and Surgery"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.amsu.2021.102779"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd on behalf of IJS Publishing Group Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Abdulamir",
"given": "Ahmed S.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gorial",
"given": "Faiq I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saadi",
"given": "Sattar Jabar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maulood",
"given": "Mohammed Fauzi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashim",
"given": "Hashim Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alnuaimi",
"given": "Ahmed Sameer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "abdulrrazaq",
"given": "Manal K.",
"sequence": "additional"
}
],
"container-title": "Annals of Medicine & Surgery",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
4
]
],
"date-time": "2021-09-04T06:01:57Z",
"timestamp": 1630735317000
},
"deposited": {
"date-parts": [
[
2023,
3,
12
]
],
"date-time": "2023-03-12T23:06:36Z",
"timestamp": 1678662396000
},
"indexed": {
"date-parts": [
[
2023,
11,
17
]
],
"date-time": "2023-11-17T06:42:51Z",
"timestamp": 1700203371774
},
"is-referenced-by-count": 15,
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 1,
"start": {
"date-parts": [
[
2021,
9,
2
]
],
"date-time": "2021-09-02T00:00:00Z",
"timestamp": 1630540800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2049080121007299?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2049080121007299?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.lww.com/10.1016/j.amsu.2021.102779",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1016/j.amsu.2021.102779"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Surgery"
],
"subtitle": [],
"title": "A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "69"
}