Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.44942, NCT04399356, Feb 2022
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
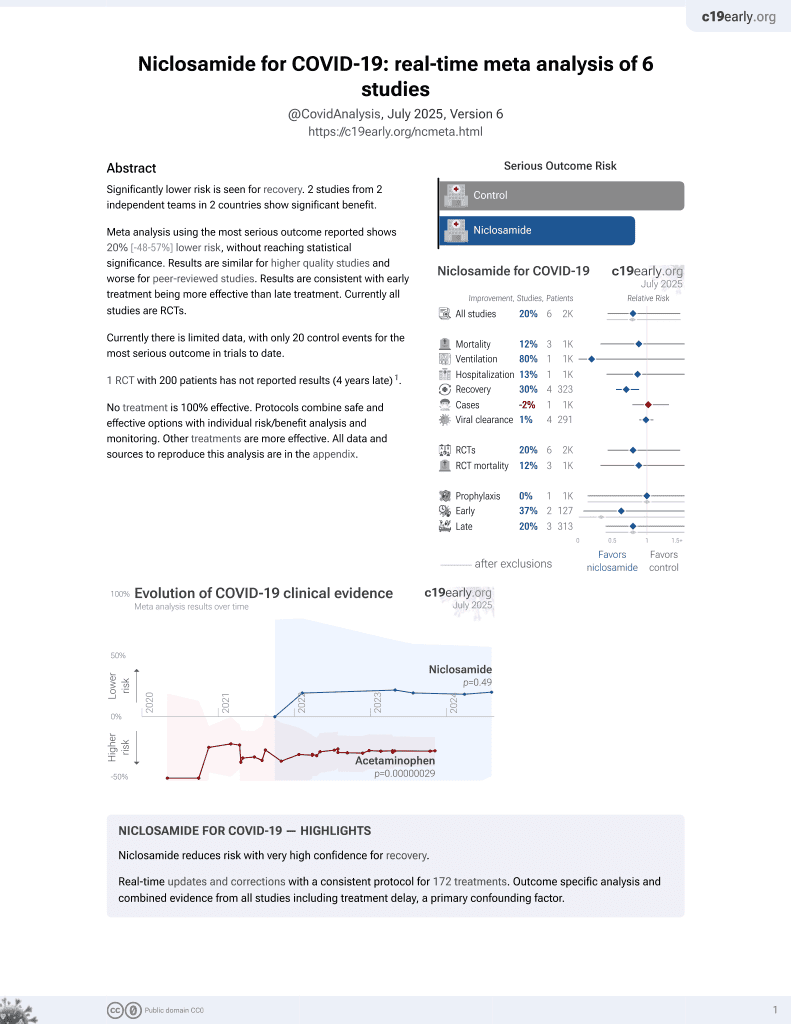
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT with 73 mild to moderate outpatients, showing faster recovery and improved viral clearance with niclosamide, without statistical significance. Greater improvements in recovery were seen for high-risk patients, again without statistical significance. The study was underpowered due to decreased enrollment related to falling COVID-19 cases.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of severe case, 66.3% lower, RR 0.34, p = 1.00, treatment 0 of 33 (0.0%), control 1 of 34 (2.9%), NNT 34, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
recovery time, 17.8% lower, relative time 0.82, p = 0.28, treatment mean 12.01 (±9.35) n=33, control mean 14.61 (±9.98) n=34.
|
|
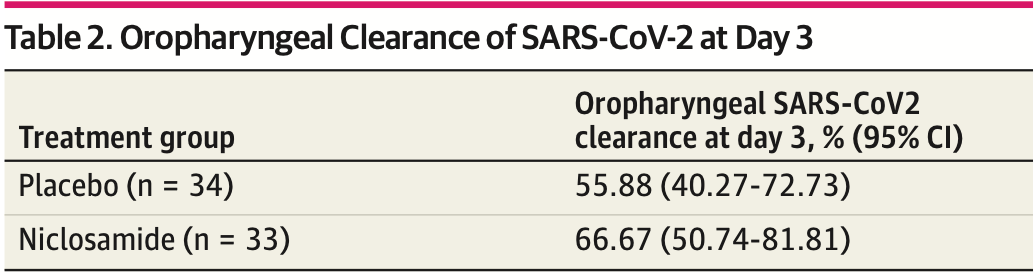
risk of no viral clearance, 24.4% lower, RR 0.76, p = 0.45, treatment 11 of 33 (33.3%), control 15 of 34 (44.1%), NNT 9.3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cairns et al., 9 Feb 2022, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, 9 authors, study period July 2021 - September 2021, trial NCT04399356 (history).
Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19
JAMA Network Open, doi:10.1001/jamanetworkopen.2021.44942
IMPORTANCE Oral anthelmintic niclosamide has potent in vitro antiviral activity against SARS-CoV-2. Repurposed niclosamide could be a safe and efficacious COVID-19 therapy. OBJECTIVE To investigate whether niclosamide decreased SARS-CoV-2 shedding and duration of symptoms among patients with mild to moderate COVID-19. DESIGN, SETTING, AND PARTICIPANTS This randomized, placebo-controlled clinical trial enrolled individuals testing positive for SARS-CoV-2 by polymerase chain reaction with mild to moderate symptoms of COVID. All trial participants, investigators, staff, and laboratory personnel were kept blind to participant assignments. Enrollment was among individuals reporting at Tufts Medical Center and Wellforce Network in Massachusetts for outpatient COVID-19 testing. The trial opened to accrual on October 1, 2020; the last participant enrolled on April 20, 2021. Trial exclusion criteria included hospitalization at time of enrollment or use of any experimental treatment for COVID-19, including vaccination. Enrollment was stopped before attaining the planned sample size when COVID-19 diagnoses decreased precipitously in Massachusetts. Data were analyzed from July through September 2021. INTERVENTIONS In addition to receiving current standard of care, participants were randomly assigned on a 1:1 basis to receive niclosamide 2 g by mouth daily for 7 days or identically labeled placebo at the same dosing schedule.
MAIN OUTCOMES AND MEASURES Oropharyngeal and fecal samples were self-collected for viral shedding measured by reverse-transcriptase-polymerase-chain-reaction on days 3, 7, 10, and 14, and an additional fecal sample was collected on day 21. A telehealth platform was developed to conduct remote study visits, monitor symptoms, and coordinate sample collection via couriers. The primary end point was the proportion of participants with viral clearance in respiratory samples at day 3 based on the intention-to-treat sample. Mean times to viral clearance and symptom resolution were calculated as restricted mean survival times and accounted for censored observations. RESULTS Among 73 participants, 36 individuals were enrolled and randomized to niclosamide and 37 individuals to placebo. Participant characteristics were similar across treatment groups; among 34 patients receiving placebo and 33 patients receiving niclosamide in the intention-to-treat sample, mean (SD) age was 36.0 (13.3) years vs 36.8 (12.9) years and there were 21 (61.8%) men vs 20 (60.6%) men. The overall mean (SD) age was 36.4 (13.0) years. For the primary end point, 66.67% (95% CI, 50.74% to 81.81%) of participants receiving niclosamide and 55.88% (95% CI, 40.27% to (continued) Key Points Question Does oral niclosamide decrease the contagious period as determined by SARS-CoV-2 shedding among patients with mild to moderate COVID-19? Findings In this randomized clinical trial that included 73 adults with mild to moderate COVID-19, the proportion of participants achieving..
Conflict of Interest Disclosures: Dr Golan reported receiving personal fees from Pfizer, Merck, and Appili Therapeutics outside the submitted work. Dr Beaulac reported receiving personal fees from Astellas Pharma and Allergan-AbbVie and grants from Diatherix outside the submitted work. No other disclosures were reported.
References
Alcendor, Racial disparities-associated COVID-19 mortality among minority populations in the US, J Clin Med, doi:10.3390/jcm9082442
Andrasfay, Goldman, Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2014746118
Braga, Ali, Secco, Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature, doi:10.1038/s41586-021-03491-6
Burock, Daum, Keilholz, Neumann, Walther et al., Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: the NIKOLO trial, BMC Cancer, doi:10.1186/s12885-018-4197-9
Burock, Daum, Tröger, Niclosamide a new chemotherapy agent: pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial, J Clin Oncol, doi:10.1200/JCO.2018.36.15_suppl.e14536
Cabrita, Benedetto, Schreiber, Kunzelmann, Niclosamide repurposed for the treatment of inflammatory airway disease, JCI Insight, doi:10.1172/jci.insight.128414
Cairns, Boorgu, Levin, Kaplan, Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells, Biol Open, doi:10.1242/bio.031807
Chan, Lee, Chan, Seasonal influenza A virus in feces of hospitalized adults, Emerg Infect Dis, doi:10.3201/eid1711.110205
Chang, Yeh, Lin, Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats, J Food Drug Anal, doi:10.38212/2224-6614.2464
Chen, Mook, Jr, Premont, Wang, Niclosamide: beyond an antihelminthic drug, Cell Signal, doi:10.1016/j.cellsig.2017.04.001
Fajnzylber, Regan, Coxen, Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Guo, Tao, Flavell, Zhu, Potential intestinal infection and faecal-oral transmission of SARS-CoV-2, Nat Rev Gastroenterol Hepatol, doi:10.1038/s41575-021-00416-6
Hacisuleyman, Hale, Saito, Vaccine breakthrough infections with SARS-CoV-2 variants, N Engl J Med, doi:10.1056/NEJMoa2105000
Jeon, Ko, Lee, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Jeon, Ko, Lee, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Jin, Lian, Hu, Epidemiological, clinical and virological characteristics of 74 cases of coronavirusinfected disease 2019 (COVID-19) with gastrointestinal symptoms, Gut, doi:10.1136/gutjnl-2020-320926
Jones, Baluja, Graham, Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19, Sci Total Environ, doi:10.1016/j.scitotenv.2020.141364
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog, doi:10.1371/journal.ppat.1002976
Kevadiya, Machhi, Herskovitz, Diagnostics for SARS-CoV-2 infections, Nat Mater, doi:10.1038/s41563-020-00906-z
Klein, Logan, Harhoff, Andersen, Analyzing survival curves at a fixed point in time, Stat Med, doi:10.1002/sim.2864
Kojima, Turner, Slepnev, Self-collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for coronavirus disease 2019 detection, Clin Infect Dis, doi:10.1093/cid/ciaa1589
Longmore, Miller, Bekkering, Diabetes and overweight/ obesity are independent, nonadditive risk factors for in-hospital severity of COVID-19: an international, multicenter retrospective meta-analysis, Diabetes Care, doi:10.2337/dc20-2676
Mccaw, Tian, Vassy, How to quantify and interpret treatment effects in comparative clinical studies of COVID-19, Ann Intern Med, doi:10.7326/M20-4044
Miner, Labitzke, Liu, Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways, Front Pharmacol, doi:10.3389/fphar.2019.00051
O'dempsey, Chapter 64-helminthic infections
Parikh, Liu, Wu, Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer, Sci Rep, doi:10.1038/s41598-021-85969-x
Pedersen, Tornby, Bang, Rectally shed SARS-CoV-2 lacks infectivity: time to rethink faecal-oral transmission?, Nat Rev Gastroenterol Hepatol, doi:10.1038/s41575-021-00501-w
Pickering, Batra, Merrick, Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study, Lancet Microbe, doi:10.1016/S2666-5247(21)00143-9
Pindiprolu, Pindiprolu, Plausible mechanisms of niclosamide as an antiviral agent against COVID-19, Med Hypotheses, doi:10.1016/j.mehy.2020.109765
Polack, Thomas, Kitchin, 4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Qian, Fan, Liu, Direct evidence of active SARS-CoV-2 replication in the intestine, Clin Infect Dis, doi:10.1093/cid/ciaa925
Sekulovski, Whorton, Tanaka, Niclosamide suppresses macrophage-induced inflammation in endometriosis, Biol Reprod, doi:10.1093/biolre/ioaa010
Thatikonda, Pooladanda, Godugu, Repurposing an old drug for new use: niclosamide in psoriasis-like skin inflammation, J Cell Physiol, doi:10.1002/jcp.29413
Trinquart, Jacot, Conner, Porcher, Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials, J Clin Oncol, doi:10.1200/JCO.2015.64.2488
Weiss, Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the UK B.1.1.7 and SA B.1.351 variant, bioRxiv, doi:10.1101/2021.04.26.441457
Wu, Guo, Tang, Prolonged presence of SARS-CoV-2 viral RNA in faecal samples, Lancet Gastroenterol Hepatol, doi:10.1016/S2468-1253(20)30083-2
Wu, Jan, Chen, Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob Agents Chemother, doi:10.1128/AAC.48.7.2693-2696.2004
Xu, Lee, Wen, Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen, Nat Med, doi:10.1038/nm.4184
Zhou, Li, Liu, Infection of bat and human intestinal organoids by SARS-CoV-2, Nat Med, doi:10.1038/s41591-020-0912-6
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2021.44942",
"author": [
{
"affiliation": [
{
"name": "Department of Biomedical Engineering, Tufts University, Medford, Massachusetts"
}
],
"family": "Cairns",
"given": "Dana M.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts"
},
{
"name": "Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts"
}
],
"family": "Dulko",
"given": "Dorothy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, Massachusetts"
},
{
"name": "Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, Massachusetts"
}
],
"family": "Griffiths",
"given": "Jeffrey K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts"
},
{
"name": "Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, Massachusetts"
}
],
"family": "Golan",
"given": "Yoav",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts"
}
],
"family": "Cohen",
"given": "Theodora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts"
}
],
"family": "Trinquart",
"given": "Ludovic",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts"
}
],
"family": "Price",
"given": "Lori Lyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Emerson Hospital, Concord, Massachusetts"
}
],
"family": "Beaulac",
"given": "Kirthana R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts"
},
{
"name": "Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts"
}
],
"family": "Selker",
"given": "Harry P.",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T16:01:53Z",
"timestamp": 1644422513000
},
"deposited": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T16:02:04Z",
"timestamp": 1644422524000
},
"indexed": {
"date-parts": [
[
2024,
3,
14
]
],
"date-time": "2024-03-14T21:28:34Z",
"timestamp": 1710451714519
},
"is-referenced-by-count": 34,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
2,
9
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2788857/cairns_2022_oi_211243_1643816437.26522.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e2144942",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
2,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
9
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/j.cellsig.2017.04.001",
"article-title": "Niclosamide: beyond an antihelminthic drug.",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "Cell Signal",
"key": "zoi211243r1",
"volume": "41",
"year": "2018"
},
{
"DOI": "10.1128/AAC.48.7.2693-2696.2004",
"article-title": "Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide.",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "2693",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "zoi211243r2",
"volume": "48",
"year": "2004"
},
{
"article-title": "Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats.",
"author": "Chang",
"issue": "4",
"journal-title": "J Food Drug Anal",
"key": "zoi211243r3",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs.",
"author": "Jeon",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "zoi211243r4",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1002976",
"article-title": "Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects.",
"author": "Jurgeit",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "PLoS Pathog",
"key": "zoi211243r6",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.1038/nm.4184",
"article-title": "Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen.",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "1101",
"issue": "10",
"journal-title": "Nat Med",
"key": "zoi211243r7",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"article-title": "Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia.",
"author": "Braga",
"doi-asserted-by": "publisher",
"first-page": "88",
"issue": "7861",
"journal-title": "Nature",
"key": "zoi211243r8",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.1093/biolre/ioaa010",
"article-title": "Niclosamide suppresses macrophage-induced inflammation in endometriosis.",
"author": "Sekulovski",
"doi-asserted-by": "publisher",
"first-page": "1011",
"issue": "5",
"journal-title": "Biol Reprod",
"key": "zoi211243r9",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1002/jcp.v235.6",
"article-title": "Repurposing an old drug for new use: niclosamide in psoriasis-like skin inflammation.",
"author": "Thatikonda",
"doi-asserted-by": "publisher",
"first-page": "5270",
"issue": "6",
"journal-title": "J Cell Physiol",
"key": "zoi211243r10",
"volume": "235",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.128414",
"article-title": "Niclosamide repurposed for the treatment of inflammatory airway disease.",
"author": "Cabrita",
"doi-asserted-by": "crossref",
"issue": "15",
"journal-title": "JCI Insight",
"key": "zoi211243r11",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.3389/fphar.2019.00051",
"article-title": "Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways.",
"author": "Miner",
"doi-asserted-by": "publisher",
"first-page": "51",
"journal-title": "Front Pharmacol",
"key": "zoi211243r12",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/s41598-021-85969-x",
"article-title": "Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer.",
"author": "Parikh",
"doi-asserted-by": "publisher",
"first-page": "6377",
"issue": "1",
"journal-title": "Sci Rep",
"key": "zoi211243r13",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1200/JCO.2018.36.15_suppl.e14536",
"article-title": "Niclosamide a new chemotherapy agent: pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial.",
"author": "Burock",
"doi-asserted-by": "crossref",
"issue": "15_suppl",
"journal-title": "J Clin Oncol",
"key": "zoi211243r14",
"volume": "36",
"year": "2018"
},
{
"DOI": "10.1186/s12885-018-4197-9",
"article-title": "Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: the NIKOLO trial.",
"author": "Burock",
"doi-asserted-by": "publisher",
"first-page": "297",
"issue": "1",
"journal-title": "BMC Cancer",
"key": "zoi211243r15",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1136/gutjnl-2020-320926",
"article-title": "Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms.",
"author": "Jin",
"doi-asserted-by": "publisher",
"first-page": "1002",
"issue": "6",
"journal-title": "Gut",
"key": "zoi211243r16",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1016/S2468-1253(20)30083-2",
"article-title": "Prolonged presence of SARS-CoV-2 viral RNA in faecal samples.",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "434",
"issue": "5",
"journal-title": "Lancet Gastroenterol Hepatol",
"key": "zoi211243r17",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs.",
"author": "Jeon",
"doi-asserted-by": "publisher",
"first-page": "e00819",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "zoi211243r18",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109765",
"article-title": "Plausible mechanisms of niclosamide as an antiviral agent against COVID-19.",
"author": "Pindiprolu",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses",
"key": "zoi211243r19",
"volume": "140",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1589",
"article-title": "Self-collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for coronavirus disease 2019 detection.",
"author": "Kojima",
"doi-asserted-by": "publisher",
"first-page": "e3106",
"issue": "9",
"journal-title": "Clin Infect Dis",
"key": "zoi211243r20",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1002/(ISSN)1097-0258",
"article-title": "Analyzing survival curves at a fixed point in time.",
"author": "Klein",
"doi-asserted-by": "publisher",
"first-page": "4505",
"issue": "24",
"journal-title": "Stat Med",
"key": "zoi211243r21",
"volume": "26",
"year": "2007"
},
{
"DOI": "10.1200/JCO.2015.64.2488",
"article-title": "Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials.",
"author": "Trinquart",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "15",
"journal-title": "J Clin Oncol",
"key": "zoi211243r22",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.7326/M20-4044",
"article-title": "How to quantify and interpret treatment effects in comparative clinical studies of COVID-19.",
"author": "McCaw",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "zoi211243r23",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality.",
"author": "Fajnzylber",
"doi-asserted-by": "publisher",
"first-page": "5493",
"issue": "1",
"journal-title": "Nat Commun",
"key": "zoi211243r24",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.2337/dc20-2676",
"article-title": "Diabetes and overweight/obesity are independent, nonadditive risk factors for in-hospital severity of COVID-19: an international, multicenter retrospective meta-analysis.",
"author": "Longmore",
"doi-asserted-by": "publisher",
"first-page": "1281",
"issue": "6",
"journal-title": "Diabetes Care",
"key": "zoi211243r25",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1038/s41575-021-00416-6",
"article-title": "Potential intestinal infection and faecal-oral transmission of SARS-CoV-2.",
"author": "Guo",
"doi-asserted-by": "publisher",
"first-page": "269",
"issue": "4",
"journal-title": "Nat Rev Gastroenterol Hepatol",
"key": "zoi211243r27",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1038/s41575-021-00501-w",
"article-title": "Rectally shed SARS-CoV-2 lacks infectivity: time to rethink faecal-oral transmission?",
"author": "Pedersen",
"doi-asserted-by": "publisher",
"first-page": "669",
"issue": "9",
"journal-title": "Nat Rev Gastroenterol Hepatol",
"key": "zoi211243r28",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.scitotenv.2020.141364",
"article-title": "Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19.",
"author": "Jones",
"doi-asserted-by": "crossref",
"journal-title": "Sci Total Environ",
"key": "zoi211243r29",
"volume": "749",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0912-6",
"article-title": "Infection of bat and human intestinal organoids by SARS-CoV-2.",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1077",
"issue": "7",
"journal-title": "Nat Med",
"key": "zoi211243r30",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa925",
"article-title": "Direct evidence of active SARS-CoV-2 replication in the intestine.",
"author": "Qian",
"doi-asserted-by": "publisher",
"first-page": "361",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "zoi211243r31",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41563-020-00906-z",
"article-title": "Diagnostics for SARS-CoV-2 infections.",
"author": "Kevadiya",
"doi-asserted-by": "publisher",
"first-page": "593",
"issue": "5",
"journal-title": "Nat Mater",
"key": "zoi211243r32",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/S2666-5247(21)00143-9",
"article-title": "Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study.",
"author": "Pickering",
"doi-asserted-by": "publisher",
"first-page": "e461",
"issue": "9",
"journal-title": "Lancet Microbe",
"key": "zoi211243r33",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.3201/eid1711.110205",
"article-title": "Seasonal influenza A virus in feces of hospitalized adults.",
"author": "Chan",
"doi-asserted-by": "publisher",
"first-page": "2038",
"issue": "11",
"journal-title": "Emerg Infect Dis",
"key": "zoi211243r34",
"volume": "17",
"year": "2011"
},
{
"DOI": "10.3390/jcm9082442",
"article-title": "Racial disparities-associated COVID-19 mortality among minority populations in the US.",
"author": "Alcendor",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "J Clin Med",
"key": "zoi211243r35",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2014746118",
"article-title": "Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations.",
"author": "Andrasfay",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "zoi211243r36",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1242/bio.031807",
"article-title": "Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells.",
"author": "Cairns",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Biol Open",
"key": "zoi211243r38",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine.",
"author": "Polack",
"doi-asserted-by": "publisher",
"first-page": "2603",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "zoi211243r39",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2105000",
"article-title": "Vaccine breakthrough infections with SARS-CoV-2 variants.",
"author": "Hacisuleyman",
"doi-asserted-by": "publisher",
"first-page": "2212",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "zoi211243r40",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/B978-0-7020-4064-1.00064-6",
"author": "O'Dempsey",
"doi-asserted-by": "crossref",
"edition": "9th ed",
"first-page": "842",
"key": "zoi211243r37",
"volume-title": "Antibiotic and Chemotherapy",
"year": "2010"
},
{
"DOI": "10.1101/2021.04.26.441457",
"doi-asserted-by": "crossref",
"key": "zoi211243r5",
"unstructured": "Weiss, A., ? Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the UK B.1.1.7 and SA B.1.351 variant.? bioRxiv, 2021: p. 2021.04.26.441457. doi:10.1101/2021.04.26.441457?"
},
{
"key": "zoi211243r26",
"unstructured": "Centers for Disease Control and Prevention. Scientific brief: SARS-CoV-2 transmission. Updated May 7, 2021. Accessed December 7, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2788857"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Phase 2 Randomized Clinical Trial"
],
"title": "Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients With Mild to Moderate COVID-19",
"type": "journal-article",
"volume": "5"
}