A Phase III, Randomized, Placebo-controlled, Clinical Trial to Evaluate the Efficacy and Safety of Co-administered Niclosamide in Patients Treated With an Established Regimen for Novel Coronavirus Infectious Disease (COVID-19)
et al., NCT04558021, NICLONEX, NCT04558021, Feb 2021
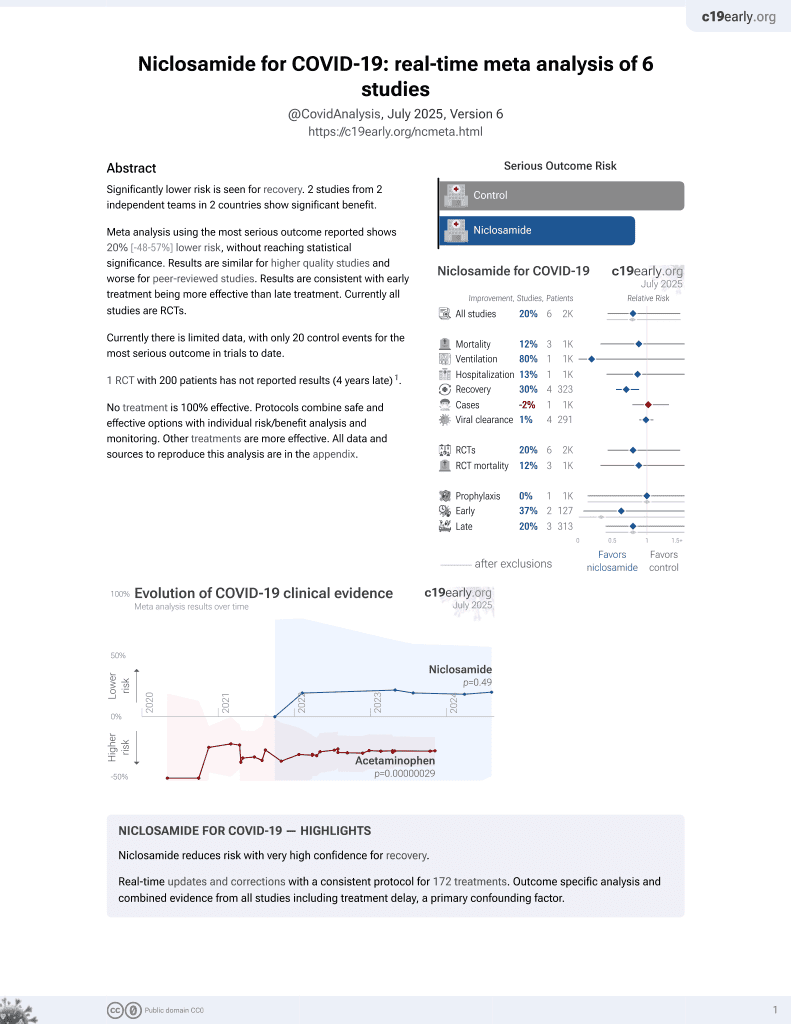
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Estimated 200 patient niclosamide late treatment RCT with results not reported over 5 years after estimated completion.
Erenmemisoglu et al., 14 Feb 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Turkey, trial NCT04558021 (history) (NICLONEX).
Contact: erenmemis@gmail.com, ayseozlem_ornek@hotmail.com, ikaraoglan10@hotmail.com.