Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-10584-4, PROTECT-V, NCT04870333, Jul 2023 (preprint)
56th treatment shown to reduce risk in
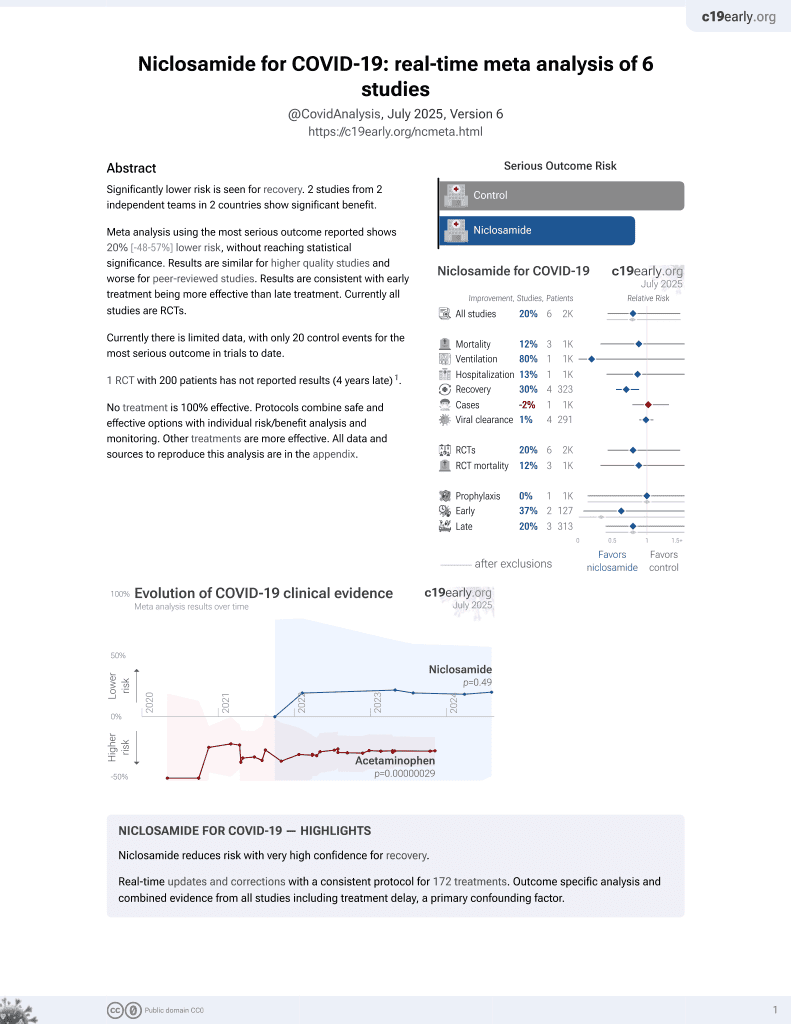
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 1,651 patients with kidney disease showing no significant difference in symptomatic COVID-19, hospitalization, or mortality with intranasal niclosamide compared to placebo. The UNI911 nasal spray had very poor adherence and a higher withdrawal rate (40% vs. 23.8% for placebo), partially due to local nasal and upper airway irritation.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 0.1% lower, RR 1.00, p = 1.00, treatment 2 of 826 (0.2%), control 2 of 825 (0.2%), NNT 340725.
|
|
risk of mechanical ventilation, 80.0% lower, RR 0.20, p = 0.25, treatment 0 of 826 (0.0%), control 2 of 825 (0.2%), NNT 412, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 13.4% lower, RR 0.87, p = 0.71, treatment 13 of 826 (1.6%), control 15 of 825 (1.8%), NNT 409.
|
|
risk of symptomatic case, 2.0% higher, HR 1.02, p = 0.89, treatment 103 of 826 (12.5%), control 133 of 825 (16.1%), adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Humphrey et al., 26 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, median age 55.9, 21 authors, study period 19 February, 2021 - 28 November, 2022, trial NCT04870333 (history) (PROTECT-V).
Contact: rms50@cam.ac.uk.
Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial
BMC Infectious Diseases, doi:10.1186/s12879-025-10584-4
Purpose Despite vaccination, many patients remain vulnerable to COVID-19 infection and poorer outcomes, because of underlying health conditions resulting in sub-optimal vaccine responses. This study aims to demonstrate whether intranasal niclosamide confers additional protection against COVID-19 infection above standard preventative measures including vaccination. Methods PROTECT-V (PROphylaxis for paTiEnts at risk of COVID-19 infecTion) is a platform trial testing multiple pre-exposure COVID-19 prophylactic agents in vulnerable patients. This paper reports results from the randomised, double blind, placebo controlled intranasal niclosamide arm. 1651 adult patients on dialysis, with a kidney transplant or renal autoimmune conditions on immunosuppression were randomised from 48 sites (37 UK; 11 Indian). Intranasal niclosamide or matched placebo was administered twice daily, for up to nine months. Primary outcome was time to symptomatic COVID-19 infection. Results 1651 patients were randomised (826 niclosamide;825 placebo) between February 2021 to November 2022. 655(39.7%) were dialysis patients, 622(37.7%) kidney transplant recipients and 374(22.7%) had renal autoimmune disease. 97.5% patients in the UK and 66.4% patients in India with comparable proportions in both treatment groups had received COVID-19 vaccinations. Despite no adverse safety signal, there was a high withdrawal rate (40% niclosamide;23.8% placebo) due to local upper airway irritation leading to a significantly shorter treatment duration in the niclosamide group). Symptomatic COVID-19 infection during study treatment was observed in 103 patients in the niclosamide group and 133 in the placebo group (estimated hazard ratio 1.02(95%CI 0.79-1.32)).
Conclusion Intranasal niclosamide did not reduce risk of symptomatic COVID-19 infection in this cohort compared to placebo.
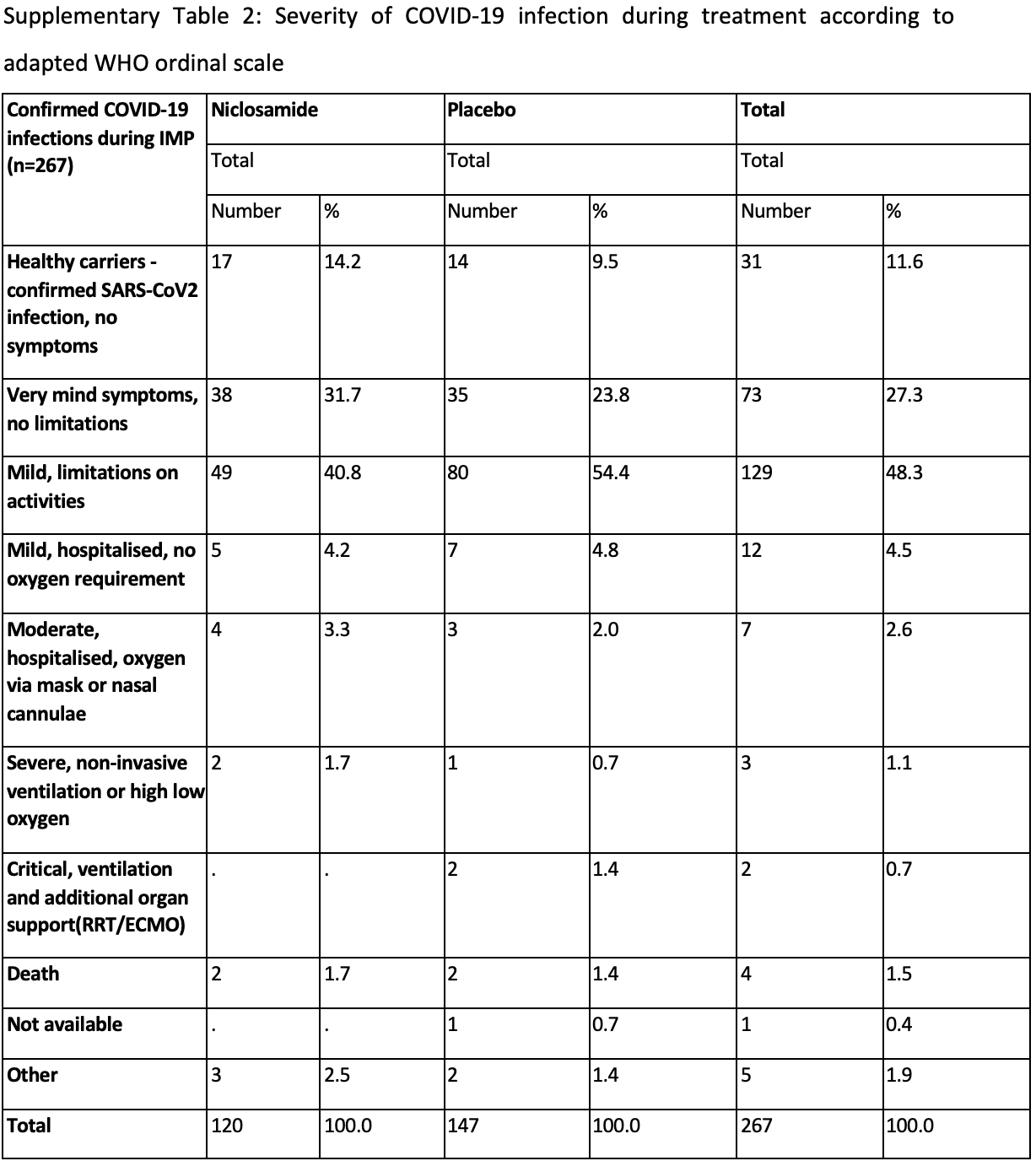
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1186/ s12879-025-10584-4. Supplementary Material 1. Table S1 . Effect of baseline covariates on development of symptomatic COVID-19 infection. Table S2 : Severity of COVID-19 infection during treatment according to adapted WHO ordinal scale. Table S3 . Proportion of patient reporting moderate symptoms, whilst receiving treatment, at any time point from starting treatment. Table S4 . Serious adverse events reported during the trial in subjects who received at least one dose of Investigational Medicinal Product (IMP). SOC-System Organ Class; PT -Preferred Term. Figure S1 . Kaplan-Meier plots on time on trial treatment without confirmed symptomatic COVID-19 infection by treatment allocation. Figure S2 . Forest plot of time to confirmed symptomatic COVID-19 infection by baseline characteristics. p value of the associated interaction with the treatment allocation is presented next to each of the subgroup headings.
Role of the funding source The PROTECT-V study was funded by LifeArc, Addenbrooke's Charitable Trust and Kidney Research UK. Intranasal niclosamide (UNI911) and additional funding for the niclosamide arm, both in the UK and India, was provided by UNION therapeutics A/S. This was an academic initiated study, and decisions regarding the design, conduct and reporting of the study rested with the study team and sponsors (Cambridge University Hospitals..
References
Backer, Sjöbring, Sonne, Weiss, Hostrup et al., A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19, Lancet Reg Heal -Eur
Bingham, Looney, Deodhar, Halsey, Greenwald et al., Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial, Arthritis Rheum
Braga, Ali, Secco, Chiavacci, Neves et al., Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature
Caillard, Thaunat, COVID-19 vaccination in kidney transplant recipients, Nat Rev Nephrol
Fine, Gray, A Proportional Hazards Model for the Subdistribution of a Competing Risk, J Am Stat Assoc, doi:10.1080/01621459.1999.10474144
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nat Commun
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Guy, Thomas, None
Humphrey, Dosanjh, Hiemstra, Richter, Chen-Xu et al., PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V), Trials, doi:10.1186/s13063-023-07128-z
Jeon, Ko, Lee, Choi, Byun et al., Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Krueger, Ison, Ghossein, Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation, Am J Kidney Dis
Levin, Ustianowski, De, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, N Engl J Med
Pindiprolu, Pindiprolu, Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19, Med Hypotheses
Pinto, Park, Beltramello, Walls, Tortorici et al., Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Queen, King's Lynn, UK 52
Singh, Weiss, Goodman, Fisk, Kulkarni et al., Niclosamide-A promising treatment for COVID-19, Br J Pharmacol, doi:10.1111/bph.15843
Smith, Cooper, Doffinger, Stacey, Al-Mohammad et al., SARS-COV-2 vaccine responses in renal patient populations, BMC Nephrol, doi:10.1186/s12882-022-02792-w
Van Assen, Holvast, Benne, Posthumus, Van Leeuwen et al., Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab, Arthritis Rheum, doi:10.1002/art.25033
Weiss, Hendrickx, Stensgaard, Jellingsø, Sommer, Kidney Transplant and Dialysis Patients Remain at Increased Risk for Succumbing to COVID-19, Transplantation, doi:10.1097/TP.0000000000004462
Weiss, Touret, Baronti, Gilles, Hoen et al., Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2), PLoS One, doi:https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0260958
DOI record:
{
"DOI": "10.1186/s12879-025-10584-4",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-025-10584-4",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Purpose</jats:title>\n <jats:p>Despite vaccination, many patients remain vulnerable to COVID-19 infection and poorer outcomes, because of underlying health conditions resulting in sub-optimal vaccine responses. This study aims to demonstrate whether intranasal niclosamide confers additional protection against COVID-19 infection above standard preventative measures including vaccination.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>PROTECT-V (PROphylaxis for paTiEnts at risk of COVID-19 infecTion) is a platform trial testing multiple pre-exposure COVID-19 prophylactic agents in vulnerable patients. This paper reports results from the randomised, double blind, placebo controlled intranasal niclosamide arm.</jats:p>\n <jats:p>1651 adult patients on dialysis, with a kidney transplant or renal autoimmune conditions on immunosuppression were randomised from 48 sites (37 UK; 11 Indian). Intranasal niclosamide or matched placebo was administered twice daily, for up to nine months. Primary outcome was time to symptomatic COVID-19 infection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>1651 patients were randomised (826 niclosamide;825 placebo) between February 2021 to November 2022. 655(39.7%) were dialysis patients, 622(37.7%) kidney transplant recipients and 374(22.7%) had renal autoimmune disease. 97.5% patients in the UK and 66.4% patients in India with comparable proportions in both treatment groups had received COVID-19 vaccinations. Despite no adverse safety signal, there was a high withdrawal rate (40% niclosamide;23.8% placebo) due to local upper airway irritation leading to a significantly shorter treatment duration in the niclosamide group). Symptomatic COVID-19 infection during study treatment was observed in 103 patients in the niclosamide group and 133 in the placebo group (estimated hazard ratio 1.02(95%CI 0.79–1.32)).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Intranasal niclosamide did not reduce risk of symptomatic COVID-19 infection in this cohort compared to placebo.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Trial Registration</jats:title>\n <jats:p>This study is registered with ClinicalTrials.gov: NCT04870333 (submitted 01/03/2021; posted 03/05/2021), EudraCT: 2020–004144-28 and the Clinical Trials Registry of India (CTRI):#CTRI/2022/03/040802.</jats:p>\n </jats:sec>",
"alternative-id": [
"10584"
],
"article-number": "204",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "18 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "31 January 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "11 February 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approvals and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The protocol was approved by the UK Medicines and Healthcare Products Regulatory Agency, South Central Berkshire Research Ethics Committee (REC Reference 20/SC/0403) in the UK and the Central Drugs Standard Control Organisation, India and the Ethics Committees of all participating sites in India."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All authors have completed the Unified Competing Interest Form (available on request from the corresponding author) and declare the following: MCX has received PhD funding support from GlaxoSmithKline. ID has research grants from Sanofi, Baxter and NIHR CRN West Midlands; honoraria from GSK, Sanofi, Vifor and AstraZeneca; support to attend meetings from GlaxoSmithKline; and sits on DSMB/advisory boards for GlaxoSmithKline and Vifor. DD has received research grants from GlaxoSmithKline; sites on DSMB/advisory boards for Gilead Sciences Inc and Synairgen Plc and since May 2023 is an employee of AstraZeneca. MJ, PS and AWC are employees of Union therapeutics A/S. MLJ is an employee of Union therapeutics A/S and sits on the advisory board for Radiometer and board of directors for HEDIA. MAOS is a co-founder and shareholder at Union therapeutics A/S and a co-inventor on patents and patent applications around the use of niclosamide to treat COVID-19. VJ has received consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Bayer, Vera, Visterra, Biocryst, AstraZeneca; honoraria from Baxter Healthcare and GlaxoSmithKline; and sits on DSMB/advisory boards for Zydus Lifesciences. TFH is and employee and shareholder at GlaxoSmithKline and is an ICH E20 working Group Member. RMS has received research grants from LifeArc, Kidney Research UK, Addenbrooke’s Charitable Trust, Union therapeutics A/S and GlaxoSmithKline for the PROTECT-V trial. TJLH, WQ, FD, RA, TH, LS, AB, NB, JRB have no conflicts of interest to declare."
}
],
"author": [
{
"affiliation": [],
"family": "Humphrey",
"given": "Toby J. L.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Qian",
"given": "Wendi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen-Xu",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dowling",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gatley",
"given": "Katrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adhikari",
"given": "Rakshya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hensman",
"given": "Tracey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stockley",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bassi",
"given": "Abhinav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bathla",
"given": "Nikita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dasgupta",
"given": "Indranil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dosanjh",
"given": "Davinder P. S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jellingsø",
"given": "Mads",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sørensen",
"given": "Per",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Morten Lind",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Callesen",
"given": "Anne Weibel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bradley",
"given": "John R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jha",
"given": "Vivekanand",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sommer",
"given": "Morten O. A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiemstra",
"given": "Thomas F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Rona M.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "PROTECT-V consortium",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alchi",
"given": "Bassam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alejmi",
"given": "Abdulfattah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basu",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bebb",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bell",
"given": "Samira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhargava",
"given": "Anudita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhandari",
"given": "Sunil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bingham",
"given": "Coralie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bramham",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caskey",
"given": "Fergus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chand",
"given": "Sourabh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaudhry",
"given": "Dhruva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaudhury",
"given": "Arpita Ray",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chennamsetty",
"given": "Sashidhar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chitalia",
"given": "Nihil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chowdhury",
"given": "Paramit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curran",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davison",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delaney",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dey",
"given": "Vishal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dick",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eid",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El-Damanawi",
"given": "Ragada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fluck",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gama",
"given": "Rouvick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldsmith",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gkrania-Klotsas",
"given": "Effrossyni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffin",
"given": "Sian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hull",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ignatius",
"given": "Avinash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jayne",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamalnathan",
"given": "Manivarma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolhe",
"given": "Nitin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lafont",
"given": "Tanguy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lambie",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lawman",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ledson",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lightstone",
"given": "Liz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucas",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahalingasivam",
"given": "Viyaasan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mark",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McAdoo",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCafferty",
"given": "Kieran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patrick",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Narayan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pritchard",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rainone",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramachandran",
"given": "Raja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rathore",
"given": "Vinay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sahay",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salama",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saxena",
"given": "Sanjiv",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Sapna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharpe",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spencer",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taylor",
"given": "Jo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trotter",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Udayaraj",
"given": "Udaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ugni",
"given": "Shiva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wade",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wahba",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wason",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkie",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Ian",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
11
]
],
"date-time": "2025-02-11T18:29:46Z",
"timestamp": 1739298586000
},
"deposited": {
"date-parts": [
[
2025,
2,
11
]
],
"date-time": "2025-02-11T18:29:55Z",
"timestamp": 1739298595000
},
"indexed": {
"date-parts": [
[
2025,
2,
12
]
],
"date-time": "2025-02-12T05:29:44Z",
"timestamp": 1739338184146,
"version": "3.37.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
2,
11
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
11
]
],
"date-time": "2025-02-11T00:00:00Z",
"timestamp": 1739232000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
11
]
],
"date-time": "2025-02-11T00:00:00Z",
"timestamp": 1739232000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10584-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-025-10584-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10584-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
2,
11
]
]
},
"published-online": {
"date-parts": [
[
2025,
2,
11
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1097/TP.0000000000004462",
"doi-asserted-by": "crossref",
"key": "10584_CR1",
"unstructured": "Weiss A, Hendrickx R, Stensgaard E, Jellingsø M, Sommer MOA. Kidney Transplant and Dialysis Patients Remain at Increased Risk for Succumbing to COVID-19. Transplantation. 2023;107(5):1136–8. Available from: https://journals.lww.com/10.1097/TP.0000000000004462"
},
{
"DOI": "10.1053/j.ajkd.2019.06.014",
"doi-asserted-by": "crossref",
"key": "10584_CR2",
"unstructured": "Krueger KM, Ison MG, Ghossein C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am J Kidney Dis. 2020;75(3):417–25. Available from: http://www.ajkd.org/article/S0272638619308911/fulltext. [cited 2022 Oct 8]."
},
{
"DOI": "10.1002/art.25034",
"author": "CO Bingham",
"doi-asserted-by": "publisher",
"first-page": "64",
"issue": "1",
"journal-title": "Arthritis Rheum",
"key": "10584_CR3",
"unstructured": "Bingham CO, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74.",
"volume": "62",
"year": "2010"
},
{
"DOI": "10.1002/art.25033",
"doi-asserted-by": "crossref",
"key": "10584_CR4",
"unstructured": "Van Assen S, Holvast A, Benne CA, Posthumus MD, Van Leeuwen MA, Voskuyl AE, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/art.25033\n. [cited 2023 Mar 25]."
},
{
"DOI": "10.1038/s41581-021-00491-7",
"doi-asserted-by": "crossref",
"key": "10584_CR5",
"unstructured": "Caillard S, Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17(12):785–7. Available from: https://pubmed.ncbi.nlm.nih.gov/34580488/. [cited 2022 Oct 8]."
},
{
"DOI": "10.1186/s12882-022-02792-w",
"doi-asserted-by": "crossref",
"key": "10584_CR6",
"unstructured": "Smith RM, Cooper DJ, Doffinger R, Stacey H, Al-Mohammad A, Goodfellow I, et al. SARS-COV-2 vaccine responses in renal patient populations. BMC Nephrol. 2022;23(1):1–9. Available from: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-022-02792-w\n. [cited 2023 Jul 22]."
},
{
"DOI": "10.1056/NEJMoa2116620",
"doi-asserted-by": "crossref",
"key": "10584_CR7",
"unstructured": "Levin MJ, Ustianowski A, Wit S De, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022 Jun 9 [cited 2022 Oct 8];386(23):2188–200. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9069994/"
},
{
"DOI": "10.1186/s13063-023-07128-z",
"doi-asserted-by": "crossref",
"key": "10584_CR8",
"unstructured": "Humphrey TJL, Dosanjh D, Hiemstra TF, Richter A, Chen-Xu M, Qian W, et al. PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V). Trials 2023 241. 2023;24(1):1–18. Available from: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-023-07128-z\n. [cited 2023 Mar 25]."
},
{
"DOI": "10.1038/s41586-020-2349-y",
"doi-asserted-by": "crossref",
"key": "10584_CR9",
"unstructured": "Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–5. Available from: https://pubmed-ncbi-nlm-nih-gov.ezp.lib.cam.ac.uk/32422645/. [cited 2022 Feb 22]."
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "crossref",
"key": "10584_CR10",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021;385(21):1941–50. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2107934\n. [cited 2022 Oct 8]."
},
{
"DOI": "10.1038/s41586-021-03491-6",
"doi-asserted-by": "crossref",
"key": "10584_CR11",
"unstructured": "Braga L, Ali H, Secco I, Chiavacci E, Neves G, Goldhill D, et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021;594(7861):88–93. Available from: https://pubmed-ncbi-nlm-nih-gov.ezp.lib.cam.ac.uk/33827113/. [cited 2022 Feb 22]."
},
{
"DOI": "10.1128/AAC.00819-20",
"doi-asserted-by": "crossref",
"key": "10584_CR12",
"unstructured": "Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob Agents Chemother. 2020;64(7). Available from: https://journals.asm.org/doi/10.1128/AAC.00819-20"
},
{
"DOI": "10.1111/bph.15843",
"doi-asserted-by": "crossref",
"key": "10584_CR13",
"unstructured": "Singh S, Weiss A, Goodman J, Fisk M, Kulkarni S, Lu I, et al. Niclosamide—A promising treatment for COVID‐19. Br J Pharmacol. 2022;179(13):3250–67. Available from: https://onlinelibrary.wiley.com/doi/10.1111/bph.15843"
},
{
"DOI": "10.1016/j.mehy.2020.109765",
"author": "SKSS Pindiprolu",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "10584_CR14",
"unstructured": "Pindiprolu SKSS, Pindiprolu SH. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Med Hypotheses. 2020;140: 109765.",
"volume": "140",
"year": "2020"
},
{
"DOI": "10.1038/s41467-019-13659-4",
"doi-asserted-by": "crossref",
"key": "10584_CR15",
"unstructured": "Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun. 2019;10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31852899/. [cited 2022 Oct 8]."
},
{
"DOI": "10.1371/journal.pone.0260958",
"doi-asserted-by": "crossref",
"key": "10584_CR16",
"unstructured": "Weiss A, Touret F, Baronti C, Gilles M, Hoen B, Nougairède A, et al. Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2). PLoS One. 2021;16(12):e0260958. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0260958\n. [cited 2022 Oct 8]."
},
{
"DOI": "10.1016/j.lanepe.2021.100084",
"doi-asserted-by": "crossref",
"key": "10584_CR17",
"unstructured": "Backer V, Sjöbring U, Sonne J, Weiss A, Hostrup M, Johansen HK, et al. A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19. Lancet Reg Heal - Eur. 2021;4:100084. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2666776221000612"
},
{
"DOI": "10.1080/01621459.1999.10474144",
"doi-asserted-by": "crossref",
"key": "10584_CR18",
"unstructured": "Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. Available from: http://www.tandfonline.com/doi/abs/10.1080/01621459.1999.10474144"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-025-10584-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}