Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab
et al., NEJM, doi:10.1056/NEJMoa2107934 (news release 5/26/2021), NCT04545060, May 2021
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Interim results from the COMET-ICE trial showing significantly lower hospitalization with treatment. NCT04545060 (history).
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
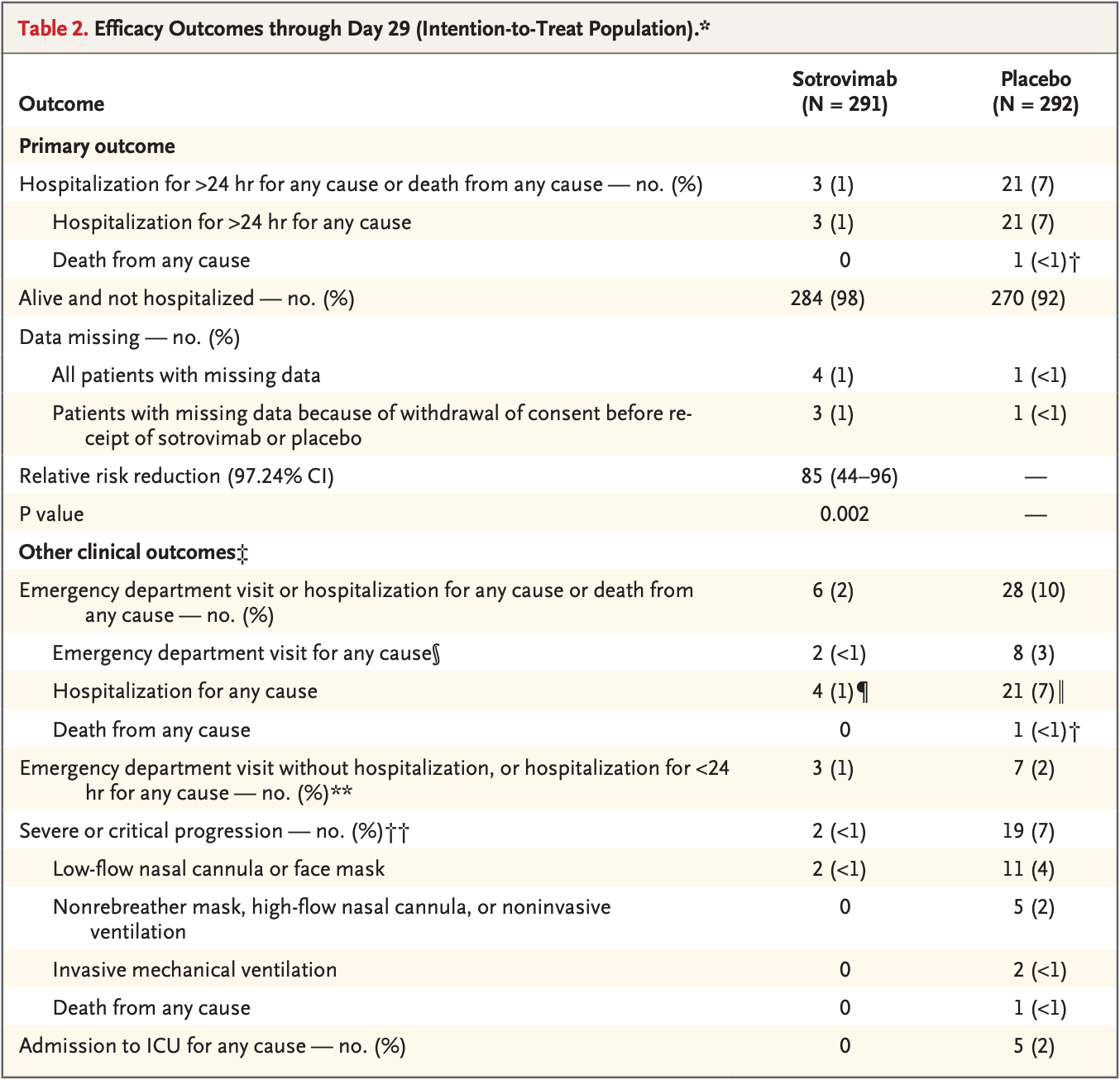
risk of death, 66.6% lower, RR 0.33, p = 1.00, treatment 0 of 291 (0.0%), control 1 of 292 (0.3%), NNT 292, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 80.0% lower, RR 0.20, p = 0.50, treatment 0 of 291 (0.0%), control 2 of 292 (0.7%), NNT 146, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 90.9% lower, RR 0.09, p = 0.06, treatment 0 of 291 (0.0%), control 5 of 292 (1.7%), NNT 58, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 80.9% lower, RR 0.19, p < 0.001, treatment 4 of 291 (1.4%), control 21 of 292 (7.2%), NNT 17.
|
|
risk of hospitalization, 85.7% lower, RR 0.14, p < 0.001, treatment 3 of 291 (1.0%), control 21 of 292 (7.2%), NNT 16, >24hrs, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Gupta et al., 26 May 2021, Double Blind Randomized Controlled Trial, USA, preprint, 22 authors, study period 27 August, 2020 - 4 March, 2021, trial NCT04545060 (history).
Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab
New England Journal of Medicine, doi:10.1056/nejmoa2107934
BACKGROUND Coronavirus disease 2019 (Covid-19) disproportionately results in hospitalization or death in older patients and those with underlying conditions. Sotrovimab is a pan-sarbecovirus monoclonal antibody that was designed to prevent progression of Covid-19 in high-risk patients early in the course of disease.
METHODS
CONCLUSIONS Among high-risk patients with mild-to-moderate Covid-19, sotrovimab reduced the risk of disease progression. No safety signals were identified. (Funded by Vir Biotechnology and GlaxoSmithKline; COMET-ICE ClinicalTrials.gov number, NCT04545060.
against the non-receptor-binding motif, which does not directly block the ACE2 receptor interaction, can be clinically therapeutic, and thus the results suggest a role for other receptors. 27 Second, because sotrovimab has potent effector function, the efficacy and absence of safety signals suggest that effector function is neither detrimental nor associated with antibody-dependent enhancement. 26 In fact, preclinical models of Covid-19 suggest that the potent effector function of this agent may be beneficial. 13, 14 The results of this interim analysis of COMET-ICE indicate that sotrovimab can be a therapeutic agent for outpatients with Covid-19. Notably, a 500-mg dose may also permit intramuscular administration, which may increase the convenience of and access to therapeutic antibody agents for patients with Covid-19. Studies are currently under way to evaluate this route of administration. Given its in vitro activity against variants of interest and concern, 14 as well as its ability to neutralize other sarbecoviruses, we speculate that sotrovimab has the potential to remain therapeutically active even as SARS-CoV-2 continues to evolve. Supported by Vir Biotechnology and GlaxoSmithKline. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
Angulo, Finelli, Swerdlow, Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys, JAMA Netw Open
Arvin, Fink, Schmid, A perspective on potential antibody-dependent enhancement of SARS-CoV-2, Nature
Cariou, Hadjadj, Wargny, Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study, Diabetologia
Cathcart, Havenar-Daughton, Lempp, The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2, doi:10.1101/2021.03.09.434607v6
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Chen, Zhang, Case, Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serumderived polyclonal antibodies, Nat Med
Focosi, Maggi, Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines, Rev Med Virol
Gaudinski, Coates, Houser, Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults, PLoS Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hwang, Shih, Cani, Group sequential designs using a family of type I error probability spending functions, Stat Med
Ko, Pegu, Rudicell, Enhanced neonatal Fc receptor function improves protection against primate SHIV infection, Nature
Lempp, Soriaga, Montiel-Ruiz, Membrane lectins enhance SARS-CoV-2 infection and influence the neutralizing activity of different classes of antibodies, doi:10.1101/2021.04.03.438258v1
Levey, Bosch, Lewis, Greene, Rogers et al., A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation, Ann Intern Med
Liang, Liang, Ou, Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19, JAMA Intern Med
Liu, Wei, Zhang, V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro, February, doi:10.1101/2021.02.16.431305v1
Mccallum, Bassi, Marco, SARS-CoV-2 immune evasion by variant B, B, doi:10.1101/2021.03.31.437925v1
Petrilli, Jones, Yang, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Reese, Iuliano, Patel, Estimated incidence of coronavirus disease 2019 (COVID-19) illness and hospitalization -United States, February-September 2020, Clin Infect Dis
Starr, Greaney, Dingens, Bloom, Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016, Cell Rep Med
Tada, Dcosta, Zhou, Vaill, Kazmierski et al., Decreased neutralization of SARS-CoV-2 global variants by therapeutic anti-spike protein monoclonal antibodies, doi:10.1101/2021.02.18.431897v1
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Zalevsky, Chamberlain, Horton, Enhanced antibody half-life improves in vivo activity, Nat Biotechnol
DOI record:
{
"DOI": "10.1056/nejmoa2107934",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2107934",
"alternative-id": [
"10.1056/NEJMoa2107934"
],
"author": [
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Gupta",
"given": "Anil",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Gonzalez-Rojas",
"given": "Yaneicy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Juarez",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Crespo Casal",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Moya",
"given": "Jaynier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Falci",
"given": "Diego R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Sarkis",
"given": "Elias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Solis",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Zheng",
"given": "Hanzhe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Scott",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Cathcart",
"given": "Andrea L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Hebner",
"given": "Christy M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Sager",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Mogalian",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Tipple",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Peppercorn",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Alexander",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Pang",
"given": "Phillip S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Free",
"given": "Almena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Brinson",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"family": "Aldinger",
"given": "Melissa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3106-1258",
"affiliation": [
{
"name": "From the Albion Finch Medical Centre, William Osler Health Centre, Toronto (A.G.); Optimus U (Y.G.-R.) and Florida International Medical Research (E.J.), Miami, Pines Care Research Center, Pembroke Pines (J.M.), and Sarkis Clinical Trials, Gainesville (E.S.) — all in Florida; Álvaro Cunqueiro Hospital, IIS Galicia Sur, Vigo, Spain (M.C.C.); Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil (D.R.F.); Centex Studies, McAllen (J. Solis), and Central Texas Clinical Research, Austin (C.B.) — both in..."
}
],
"authenticated-orcid": false,
"family": "Shapiro",
"given": "Adrienne E.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
27
]
],
"date-time": "2021-10-27T21:00:49Z",
"timestamp": 1635368449000
},
"deposited": {
"date-parts": [
[
2021,
11,
17
]
],
"date-time": "2021-11-17T23:04:52Z",
"timestamp": 1637190292000
},
"funder": [
{
"DOI": "10.13039/100004330",
"doi-asserted-by": "publisher",
"name": "Vir Biotechnology, Inc., in collaboration with GlaxoSmithKline"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T15:11:28Z",
"timestamp": 1715613088511
},
"is-referenced-by-count": 805,
"issue": "21",
"issued": {
"date-parts": [
[
2021,
11,
18
]
]
},
"journal-issue": {
"issue": "21",
"published-print": {
"date-parts": [
[
2021,
11,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
18
]
],
"date-time": "2021-11-18T00:00:00Z",
"timestamp": 1637193600000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2107934",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "1941-1950",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
11,
18
]
]
},
"published-print": {
"date-parts": [
[
2021,
11,
18
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1001/jamanetworkopen.2020.33706",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1093/cid/ciaa1780",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1136/bmj.m1966",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1001/jamainternmed.2020.2033",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1007/s00125-020-05180-x",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.15585/mmwr.mm6912e2",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "r10"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1038/s41591-021-01294-w",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1002/rmv.2231",
"doi-asserted-by": "publisher",
"key": "r16"
},
{
"DOI": "10.1016/j.xcrm.2021.100255",
"doi-asserted-by": "publisher",
"key": "r18"
},
{
"DOI": "10.1038/nature13612",
"doi-asserted-by": "publisher",
"key": "r20"
},
{
"DOI": "10.1038/nbt.1601",
"doi-asserted-by": "publisher",
"key": "r21"
},
{
"DOI": "10.1371/journal.pmed.1002493",
"doi-asserted-by": "publisher",
"key": "r22"
},
{
"DOI": "10.7326/0003-4819-130-6-199903160-00002",
"doi-asserted-by": "publisher",
"key": "r23"
},
{
"DOI": "10.1002/sim.4780091207",
"doi-asserted-by": "publisher",
"key": "r25"
},
{
"DOI": "10.1038/s41586-020-2538-8",
"doi-asserted-by": "publisher",
"key": "r26"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.05.27.21257096",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2107934"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"type": "journal-article",
"volume": "385"
}
