Niclosamide—A promising treatment for COVID‐19
et al., British Journal of Pharmacology, doi:10.1111/bph.15843, NCT04372082, Apr 2022
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
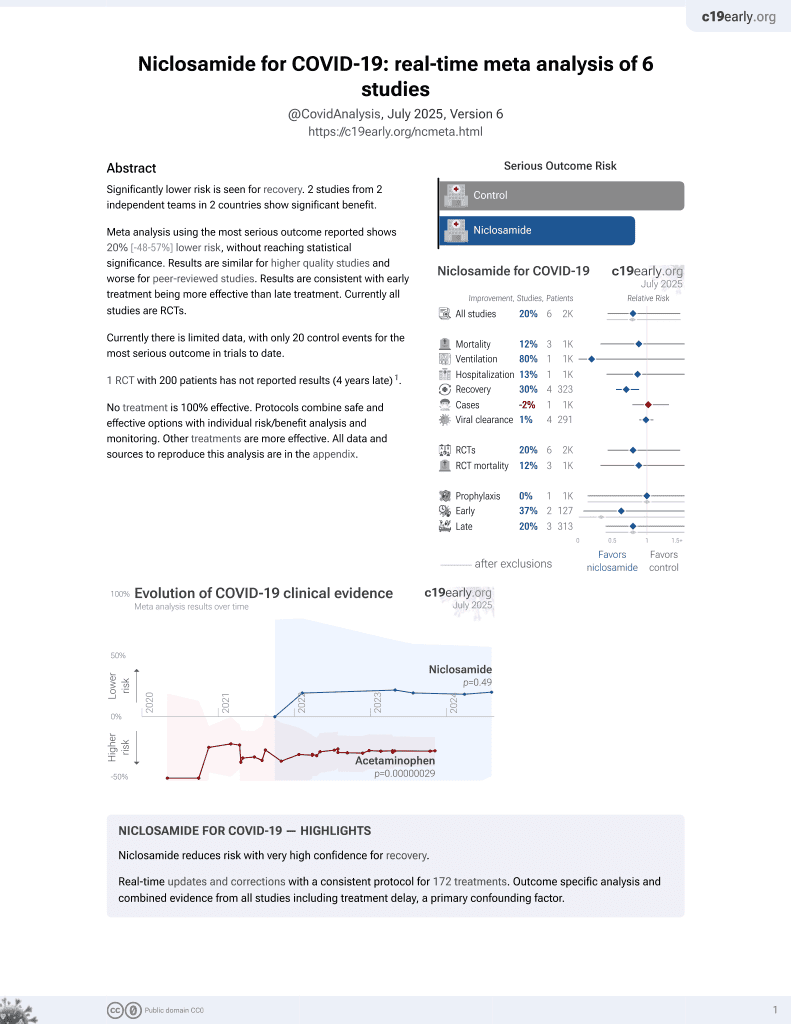
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
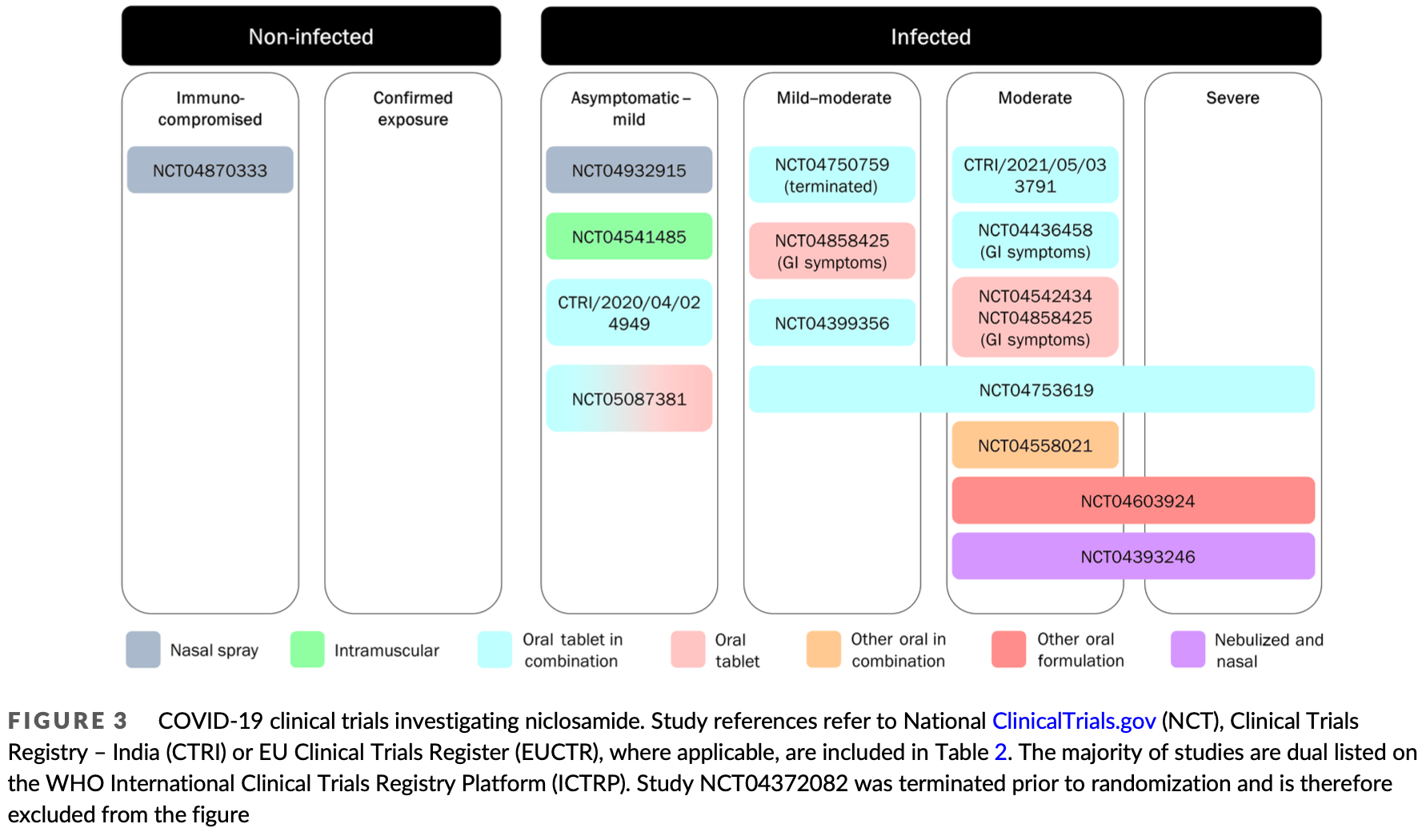
Review of niclosamide as a promising treatment for COVID-19. Authors highlight niclosamide's potent antiviral activity against SARS-CoV-2 and its pleiotropic anti-inflammatory, antibacterial, bronchodilatory and anticancer effects demonstrated in numerous preclinical and early clinical studies. The advantages and rationale for nebulized and intranasal formulations of niclosamide, which target the primary site of infection in the respiratory tract, are reviewed. An overview is provided of the 18 ongoing clinical trials investigating niclosamide as a promising candidate against SARS-CoV-2 across the full spectrum of COVID-19 disease.
1.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
2.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
3.
Ali et al., SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication, Journal of Clinical Medicine, doi:10.3390/jcm12186079.
4.
Vibhute et al., Niclosamide: a potential treatment option for COVID-19, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850.
5.
Abed et al., Evaluation the plausibility of repurpose of levamisole and niclosamide in treatment of covid-19, Journal of Pharmaceutical Technolgy, doi:10.37662/jpt.2022.999481.
6.
Cesar-Silva et al., The Endolysosomal System: The Acid Test for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23094576.
Singh et al., 11 Apr 2022, peer-reviewed, 10 authors, trial NCT04372082 (history).
Contact: jc403@cam.ac.uk.
Niclosamide—A promising treatment for COVID‐19
British Journal of Pharmacology, doi:10.1111/bph.15843
Vaccines have reduced the transmission and severity of COVID-19, but there remains a paucity of efficacious treatment for drug-resistant strains and more susceptible individuals, particularly those who mount a suboptimal vaccine response, either due to underlying health conditions or concomitant therapies. Repurposing existing drugs is a timely, safe and scientifically robust method for treating pandemics, such as COVID-19. Here, we review the pharmacology and scientific rationale for repurposing niclosamide, an anti-helminth already in human use as a treatment for COVID-19. In addition, its potent antiviral activity, niclosamide has shown pleiotropic anti-inflammatory, antibacterial, bronchodilatory and anticancer effects in numerous preclinical and early clinical studies. The advantages and rationale for nebulized and intranasal formulations of niclosamide, which target the site of the primary infection in COVID-19, are reviewed. Finally, we give an overview of ongoing clinical trials investigating niclosamide as a promising candidate against SARS-CoV-2.
AUTHOR CONTRIBUTIONS JC devised the idea for the article. SS performed the initial literature search. SS, MF, JG, SK and JG wrote the first draft. AW designed
CONFLICT OF INTEREST JC and RS acknowledge institutional grants from Union Therapeutics for the conduct of investigator initiated clinical trials of niclosamide. MS is a shareholder of UNION Therapeutics, and AW benefits from an employee incentive scheme. The other authors declare no conflict of interest.
References
Abdulamir, Gorial, Saadi, Maulood, Hashim et al., A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management, Annals of Medicine and Surgery, doi:10.1016/j.amsu.2021.102779
Al-Gareeb, Aljubory, Alkuraishy, Niclosamide as an anti-obesity drug: An experimental study, Eating and Weight Disorders, doi:10.1007/s40519-017-0373-1
Al-Gareeb, Gorial, Mahmood, Niclosamide as an adjuvant to etanercept in treatment patients with active rheumatoid arthritis: An 8-week randomized controlled pilot study, Clinical Rheumatology, doi:10.1007/s10067-018-4164-5
Al-Gareeb, Gorial, Mahmood, The anti-rheumatoid activity of niclosamide in collagen-induced arthritis in rats, Archives of Rheumatology, doi:10.5606/ArchRheumatol.2019.7100
Al-Hadiya, Niclosamide: Comprehensive profile, Profiles of Drug Substances, Excipients and Related Methodology, doi:10.1016/s0099-5428(05)32002-8
Alexander, Christopoulos, Davenport, Kelly, Mathie et al., THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein-coupled receptors, British Journal of Pharmacology, doi:10.1111/bph.15538
Alexander, Fabbro, Kelly, Mathie, Peters et al., THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors, British Journal of Pharmacology, doi:10.1111/bph.15541
Alexander, Fabbro, Kelly, Mathie, Peters et al., THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes, British Journal of Pharmacology, doi:10.1111/bph.15542
Andrews, Thyssen, Lorke, The biology and toxicology of molluscicides, Bayluscide, Pharmacology & Therapeutics, doi:10.1016/0163-7258(82)90064-x
Arend, Londoño-Joshi, Gangrade, Katre, Kurpad et al., Niclosamide and its analogs are potent inhibitors of Wnt/β-catenin, mTOR and STAT3 signaling in ovarian cancer, Oncotarget, doi:10.18632/oncotarget.13466
Arend, Londoño-Joshi, Samant, Li, Conner et al., Inhibition of Wnt/β-catenin pathway by niclosamide: A therapeutic target for ovarian cancer, Gynecologic Oncology, doi:10.1016/j.ygyno.2014.04.005
Ashburn, Thor, Drug repositioning: Identifying and developing new uses for existing drugs, Nature Reviews. Drug Discovery, doi:10.1038/nrd1468
Backer, Sjöbring, Sonne, Weiss, Hostrup et al., A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19, The Lancet Regional Health-Europe, doi:10.1016/j.lanepe.2021.100084
Barini, Miccoli, Tinarelli, Mulholland, Kadri et al., The anthelmintic drug niclosamide and its analogues activate the Parkinson's disease associated protein kinase PINK1, Chembiochem, doi:10.1002/cbic.201700500
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-Final report, The New England Journal of Medicine, doi:10.1056/NEJMoa2007764
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, The New England Journal of Medicine, doi:10.1056/NEJMoa2116044
Boyapally, Pulivendala, Bale, Godugu, Niclosamide alleviates pulmonary fibrosis in vitro and in vivo by attenuation of epithelial-to-mesenchymal transition, matrix proteins & Wnt/β-catenin signaling: A drug repurposing study, Life Sciences, doi:10.1016/j.lfs.2018.12.061
Braga, Ali, Secco, Chiavacci, Neves et al., Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spikeinduced syncytia, Nature, doi:10.1038/s41586-021-03491-6
Braga, Felix, Teixeira, Vieira, Silva-Aguiar et al., Niclosamide attenuates lung vascular remodeling in experimental pulmonary arterial hypertension, European Journal of Pharmacology, doi:10.1016/j.ejphar.2020.173438
Breckenridge, Jacob, Overcoming the legal and regulatory barriers to drug repurposing, Nature Reviews. Drug Discovery, doi:10.1038/nrd.2018.92
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patientadaptable treatment for coronavirus infections and sequalae, PLoS ONE, doi:10.1371/journal.pone.0246803
Burock, Daum, Keilholz, Neumann, Walther et al., Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial, BMC Cancer, doi:10.1186/s12885-018-4197-9
Burock, Daum, Tröger, Kim, Krüger et al., Niclosamide a new chemotherapy agent? Pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial, Journal of Clinical Oncology, doi:10.1200/JCO.2018.36.15_suppl.e14536
Cabrita, Benedetto, Schreiber, Kunzelmann, Niclosamide repurposed for the treatment of inflammatory airway disease, JCI Insight, doi:10.1172/jci.insight.128414
Cairns, Boorgu, Levin, Kaplan, Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells, Biol Open, doi:10.1242/bio.031807
Centeio, Ousingsawat, Cabrita, Schreiber, Talbi et al., Mucus release and airway constriction by TMEM16A may worsen pathology in inflammatory lung disease, International Journal of Molecular Sciences, doi:10.3390/ijms22157852
Cevik, Kuppalli, Kindrachuk, Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2, BMJ, doi:10.1136/bmj.m3862
Chang, Yeh, Lin, Chen, Yao et al., Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats, Journal of Food and Drug Analysis
Chen, Liu, Guo, Emerging coronaviruses: Genome structure, replication, and pathogenesis, Journal of Medical Virology, doi:10.1002/jmv.25681
Chen, Wei, Ma, Yang, Gill et al., Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting cell division cycle 37 signaling, Gastroenterology, doi:10.1053/j.gastro.2017.02.039
Chen, Yang, Ding, Chu, Zhang et al., Discovery of O-alkylamino tethered niclosamide derivatives as potent and orally bioavailable anticancer agents, ACS Medicinal Chemistry Letters, doi:10.1021/ml3003082
Cheng, Morales, Zhang, Mito, Tsin, Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line, PLoS ONE, doi:10.1371/journal.pone.0184324
Choi, Kim, Lee, Woo Kim, Ju Noh et al., Bioanalysis of niclosamide in plasma using liquid chromatographytandem mass and application to pharmacokinetics in rats and dogs, Journal of Chromatography B, doi:10.1016/j.jchromb.2021.122862
Chowdhury, Turner, Bentley, Das, Wu et al., Niclosamide reduces glucagon sensitivity via hepatic PKA inhibition in obese mice: Implications for glucose metabolism improvements in type 2 diabetes, Scientific Reports, doi:10.1038/srep40159
Duhm, Maul, Medenwald, Patzschke, Wegner, Radioaktive Untersuchungen mit einem neuen Molluscicid, Zeitschrift für Naturforschung Part B, doi:10.1515/znb-1961-0804
England, COVID treatment developed in the NHS saves a million lives
Fan, Xu, Files, Cirillo, Endsley et al., Dual activity of niclosamide to suppress replication of integrated HIV-1 and Mycobacterium tuberculosis (Beijing), Tuberculosis, doi:10.1016/j.tube.2019.04.008
Fehr, Perlman, Coronaviruses: An overview of their replication and pathogenesis, Methods in Molecular Biology, doi:10.1007/978-1-4939-2438-7_1
Fisk, Althage, Moosmang, Greasley, Cope et al., Endothelin antagonism and sodium glucose cotransporter 2 inhibition. A potential combination therapeutic strategy for COVID-19, Pulmonary Pharmacology & Therapeutics, doi:10.1016/j.pupt.2021.102035
Gallo, Locatello, Mazzoni, Novelli, Annunziato, The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection, Mucosal Immunology, doi:10.1038/s41385-020-00359-2
Gao, Yao, Yang, Li, From SARS to MERS: Evidence and speculation, Frontiers in Medicine, doi:10.1007/s11684-016-0466-7
Garrett, Coatsworth, Mahmud, Hamerly, Stephenson et al., Niclosamide reverses SARS-CoV-2 control of lipophagy, bioRxiv, doi:10.1101/2021.07.11.451951
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nature Communications, doi:10.1038/s41467-019-13659-4
Gassen, Papies, Bajaj, Emanuel, Dethloff et al., SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals, Nature Communications, doi:10.1038/s41467-021-24007-w
Gomes, Fernandes, Casimiro, Da Mata, Passos et al., Cathepsin L in COVID-19: From pharmacological evidences to genetics, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2020.589505
Gordon, Mouncey, Al-Beidh, Rowan, Nichol et al., Interleukin-6 receptor antagonists in critically ill patients with Covid-19, The New England Journal of Medicine, doi:10.1056/NEJMoa2100433
Gralinski, Baric, Molecular pathology of emerging coronavirus infections, The Journal of Pathology, doi:10.1002/path.4454
Group, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/s0140-6736(21)00676-0
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Effect of the neutralizing SARS-CoV-2 antibody sotrovimab in preventing progression of COVID-19: A randomized clinical trial, medRxiv, doi:10.1101/2021.11.03.21265533
Gwisai, Hollingsworth, Cowles, Tharmalingam, Mylonakis et al., Repurposing niclosamide as a versatile antimicrobial surface coating against deviceassociated, hospital-acquired bacterial infections, Biomedical Materials, doi:10.1088/1748-605X/aa7105
Gyamfi, Lee, Min, Choi, Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis, Scientific Reports, doi:10.1038/s41598-019-47707-2
Hamdoun, Jung, Efferth, Drug repurposing of the anthelmintic niclosamide to treat multidrug-resistant leukemia, Frontiers in Pharmacology, doi:10.3389/fphar.2017.00110
Han, Li, Wang, Wang, Li et al., Niclosamide induces cell cycle arrest in G1 phase in head and neck squamous cell carcinoma through let-7d/CDC34 axis, Frontiers in Pharmacology, doi:10.3389/fphar.2018.01544
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with Covid-19, The New England Journal of Medicine, doi:10.1056/NEJMoa2021436
Huang, Bosch, Li, Li, Lee et al., SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells, The Journal of Biological Chemistry, doi:10.1074/jbc.M508381200
Hurle, Yang, Xie, Rajpal, Sanseau et al., Computational drug repositioning: From data to therapeutics, Clinical Pharmacology and Therapeutics, doi:10.1038/clpt.2013.1
Imperi, Massai, Ramachandran Pillai, Longo, Zennaro et al., New life for an old drug: The anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01952-12
Jang, Lee, Hong, Song, Kim et al., Niclosamide suppresses the expansion of follicular helper T cells and alleviates disease severity in two murine models of lupus via STAT3, Journal of Translational Medicine, doi:10.1186/s12967-021-02760-2
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00819-20
Jin, Du, Xu, Deng, Liu et al., Structure of M pro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Jin, Lu, Ding, Li, Du et al., Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-κB pathway and generation of reactive oxygen species, Cancer Research, doi:10.1158/0008-5472.Can-09-3950
Jung, Nam, Oh, Jun, Ro et al., Neutralization of acidic intracellular vesicles by niclosamide inhibits multiple steps of the dengue virus life cycle in vitro, Scientific Reports, doi:10.1038/s41598-019-45095-1
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathogens, doi:10.1371/journal.ppat.1002976
Jurgeit, Moese, Roulin, Dorsch, Lötzerich et al., An RNA replication-center assay for high content image-based quantifications of human rhinovirus and coxsackievirus infections, Virology Journal, doi:10.1186/1743-422x-7-264
Kao, Huangfu, Tsai, Ho, Jhan et al., The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR, PLoS Neglected Tropical Diseases, doi:10.1371/journal.pntd.0006715
Kissler, Tedijanto, Goldstein, Grad, Lipsitch, Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period, Science, doi:10.1126/science.abb5793
Li, Brecher, Deng, Zhang, Sakamuru et al., Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction, Cell Research, doi:10.1038/cr.2017.88
Li, Yang, Han, Wen, Ma et al., Niclosamide acts as a new inhibitor of vasculogenic mimicry in oral cancer through upregulation of miR-124 and downregulation of STAT3, Oncology Reports, doi:10.3892/or.2017.6146
Li, You, Hu, Chen, Sica et al., Inhibition of STAT3 by niclosamide synergizes with erlotinib against head and neck cancer, PLoS ONE, doi:10.1371/journal.pone.0074670
Liu, Armstrong, Lou, Lombard, Cucchiara et al., Niclosamide and bicalutamide combination treatment overcomes enzalutamide-and bicalutamide-resistant prostate cancer, Molecular Cancer Therapeutics, doi:10.1158/1535-7163.Mct-16-0912
Liu, Lou, Armstrong, Zhu, Evans et al., Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition, Prostate, doi:10.1002/pros.23015
Lu, Kulkarni, Fisk, Kostapanos, Banham-Hall et al., muLTi-Arm Therapeutic study in pre-ICu patients admitted with Covid-19-Experimental drugs and mechanisms (TACTIC-E): A structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04618-2
Luo, Luo, Rong, Zhang, Chen et al., Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer, Journal for Immunotherapy of Cancer, doi:10.1186/s40425-019-0733-7
Madrid, Panchal, Warren, Shurtleff, Endsley et al., Evaluation of Ebola virus inhibitors for drug repurposing, ACS Infectious Diseases, doi:10.1021/acsinfecdis.5b00030
Mazzon, Ortega-Prieto, Imrie, Luft, Hess et al., Identification of broad-spectrum antiviral compounds by targeting viral entry, Viruses, doi:10.3390/v11020176
Miller, Becker, Grenfell, Metcalf, Disease and healthcare burden of COVID-19 in the United States, Nature Medicine, doi:10.1038/s41591-020-0952-y
Miner, Labitzke, Liu, Wang, Henckels et al., Drug repurposing: The anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways, Frontiers in Pharmacology, doi:10.3389/fphar.2019.00051
Mohammad, Abdelkhalek, Abutaleb, Seleem, Repurposing niclosamide for intestinal decolonization of vancomycinresistant enterococci, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2018.02.003
Monin, Krause, Stelling, Bocuk, Niebert et al., The anthelmintic niclosamide inhibits colorectal cancer cell lines via modulation of the canonical and noncanonical Wnt signaling pathway, The Journal of Surgical Research, doi:10.1016/j.jss.2016.03.051
Morin, Kavian, Nicco, Cerles, Chéreau et al., Niclosamide prevents systemic sclerosis in a reactive oxygen species-induced mouse model, The Journal of Immunology, doi:10.4049/jimmunol.1502482
Nosengo, Can you teach old drugs new tricks?, Nature, doi:10.1038/534314a
Parikh, Liu, Wu, Evans, Dall'era et al., Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer, Scientific Reports, doi:10.1038/s41598-021-85969-x
Paules, Marston, Fauci, Coronavirus infections-More than just the common cold, Jama, doi:10.1001/jama.2020.0757
Pearson, Hewlett, Niclosamide therapy for tapeworm infections, Annals of Internal Medicine, doi:10.7326/0003-4819-102-4-550
Prather, Maclean, Shi, Boadu, Paquet et al., Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model, Biology of Reproduction, doi:10.1095/biolreprod.116.140236
Pushpakom, Iorio, Eyers, Escott, Hopper et al., Drug repurposing: Progress, challenges and recommendations, Nature Reviews. Drug Discovery, doi:10.1038/nrd.2018.168
Rajamuthiah, Fuchs, Conery, Kim, Jayamani et al., Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus, PLoS ONE, doi:10.1371/journal.pone.0124595
Rotshild, Hirsh-Raccah, Miskin, Muszkat, Matok, Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis, Scientific Reports, doi:10.1038/s41598-021-02321-z
Schweizer, Haugk, Mckiernan, Gulati, Cheng et al., A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer, PLoS ONE, doi:10.1371/journal.pone.0198389
Schweizer, Haugk, Mckiernan, Gulati, Cheng et al., Correction: A phase I study of niclosamide in combination with enzalutamide in men with castrationresistant prostate cancer, PLoS ONE, doi:10.1371/journal.pone.0202709
Sekulovski, Whorton, Tanaka, Hirota, Shi et al., Niclosamide suppresses macrophage-induced inflammation in endometriosis †, Biology of Reproduction, doi:10.1093/biolre/ioaa010
Serrano, Apolloni, Rossi, Lattante, Sabatelli et al., The S100A4 transcriptional inhibitor niclosamide reduces proinflammatory and migratory phenotypes of microglia: Implications for amyotrophic lateral sclerosis, Cell, doi:10.3390/cells8101261
Shangguan, Liu, Ma, Qu, Lv et al., Niclosamide inhibits ovarian carcinoma growth by interrupting cellular bioenergetics, Journal of Cancer, doi:10.7150/jca.41418
Simmons, Gosalia, Rennekamp, Reeves, Diamond et al., Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry, Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.0505577102
Somersan-Karakaya, Mylonakis, Menon, Wells, Ali et al., REGEN-COV ® for treatment of hospitalized patients with Covid-19, medRxiv, doi:10.1101/2021.11.05.21265656
Stewart, Carpenter, West, Knifley, Liu et al., S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide, Oncotarget, doi:10.18632/oncotarget.8969
Suliman, Zhang, Na, Ribeiro, Zhang et al., Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family, International Journal of Molecular Medicine, doi:10.3892/ijmm.2016.2689
Sun, Zhang, Antituberculosis activity of certain antifungal and antihelmintic drugs, Tubercle and Lung Disease, doi:10.1054/tuld.1999.0212
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nature Medicine, doi:10.1038/s41591-020-0868-6
Tao, Zhang, Zeng, Shulman, Jin, Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice, Nature Medicine, doi:10.1038/nm.3699
Tharmalingam, Port, Castillo, Mylonakis, Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori, Scientific Reports, doi:10.1038/s41598-018-22037-x
Tomizawa, Shinozaki, Motoyoshi, Sugiyama, Yamamoto et al., Niclosamide suppresses hepatoma cell proliferation via the Wnt pathway, Oncotargets and Therapy, doi:10.2147/ott.s50065
Tomizawa, Shinozaki, Motoyoshi, Sugiyama, Yamamoto et al., Niclosamide suppresses migration of hepatocellular carcinoma cells and downregulates matrix metalloproteinase-9 expression, Oncology Letters, doi:10.3892/ol.2015.3789
Torres, Abercrombie, Srinivasan, Lopez-Ribot, Ramasubramanian et al., Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00377-16
Trial, Tam, Hamza, Ma, Chen et al., Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota, Nature Communications, doi:10.1038/s41467-018-07705-w
Venkatesan, Repurposing drugs for treatment of COVID-19, The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00270-8
Wang, Lu, Lin, Chin, Wu et al., Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission, Antiviral Research, doi:10.1016/j.antiviral.2016.10.003
Wang, Ren, Piao, Zhao, Osada et al., Niclosamide-induced Wnt signaling inhibition in colorectal cancer is mediated by autophagy, The Biochemical Journal, doi:10.1042/bcj20180385
Wang, Xu, Fu, Chen, Yang, The antihelminthic niclosamide inhibits cancer stemness, extracellular matrix remodeling, and metastasis through dysregulation of the nuclear β-catenin/c-Myc axis in OSCC, Scientific Reports, doi:10.1038/s41598-018-30692-3
Wang, Zhou, Xu, Shi, Zhao et al., Niclosamide inhibits cell growth and enhances drug sensitivity of hepatocellular carcinoma cells via STAT3 signaling pathway, Journal of Cancer, doi:10.7150/jca.26948
Weinbach, Garbus, Mechanism of action of reagents that uncouple oxidative phosphorylation, Nature, doi:10.1038/2211016a0
Weiss, Touret, Baronti, Gilles, Hoen et al., Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2), PLoS ONE, doi:10.1371/journal.pone.0260958
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, Journal of Medicinal Chemistry, doi:10.1021/jm070295s
Wieland, Trageser, Gogolok, Reinartz, Höfer et al., Anticancer effects of niclosamide in human glioblastoma, Clinical Cancer Research, doi:10.1158/1078-0432.Ccr-12-2895
Wu, Jan, Chen, Hsieh, Hwang et al., Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.48.7.2693-2696.2004
Wu, Zhang, Tong, Yan, Cho et al., Repurposing of niclosamide as a STAT3 inhibitor to enhance the anticancer effect of chemotherapeutic drugs in treating colorectal cancer, Life Sciences, doi:10.1016/j.lfs.2020.118522
Yang, Peng, Hsu, Lee, Wu et al., Repurposing old drugs as antiviral agents for coronaviruses, Biomedical Journal, doi:10.1016/j.bj.2020.05.003
Ye, Xiong, Yan, Xia, Song et al., The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model, PLoS ONE, doi:10.1371/journal.pone.0085887
Zeyada, Abdel-Rahman, El-Karef, Yahia, El-Sherbiny et al., Niclosamide-loaded polymeric micelles ameliorate hepatocellular carcinoma in vivo through targeting Wnt and Notch pathways, Life Sciences, doi:10.1016/j.lfs.2020.118458
Zhang, Huang, Liu, Liu, Song et al., WNT signaling underlies the pathogenesis of neuropathic pain in rodents, The Journal of Clinical Investigation, doi:10.1172/JCI65364
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zuo, Yang, Yu, Xiang, Li et al., Niclosamide enhances the cytotoxic effect of cisplatin in cisplatin-resistant human lung cancer cells via suppression of lung resistance-related protein and c-myc, Molecular Medicine Reports, doi:10.3892/mmr.2017.8301
DOI record:
{
"DOI": "10.1111/bph.15843",
"ISSN": [
"0007-1188",
"1476-5381"
],
"URL": "http://dx.doi.org/10.1111/bph.15843",
"abstract": "<jats:p>Vaccines have reduced the transmission and severity of COVID‐19, but there remains a paucity of efficacious treatment for drug‐resistant strains and more susceptible individuals, particularly those who mount a suboptimal vaccine response, either due to underlying health conditions or concomitant therapies. Repurposing existing drugs is a timely, safe and scientifically robust method for treating pandemics, such as COVID‐19. Here, we review the pharmacology and scientific rationale for repurposing niclosamide, an anti‐helminth already in human use as a treatment for COVID‐19. In addition, its potent antiviral activity, niclosamide has shown pleiotropic anti‐inflammatory, antibacterial, bronchodilatory and anticancer effects in numerous preclinical and early clinical studies. The advantages and rationale for nebulized and intranasal formulations of niclosamide, which target the site of the primary infection in COVID‐19, are reviewed. Finally, we give an overview of ongoing clinical trials investigating niclosamide as a promising candidate against SARS‐CoV‐2.</jats:p>",
"alternative-id": [
"10.1111/bph.15843"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-09-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-02-23"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-04-11"
}
],
"author": [
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine NYU School of Medicine New York New York USA"
}
],
"family": "Singh",
"given": "Shivani",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1738-0519",
"affiliation": [
{
"name": "Novo Nordisk Foundation Center for Biosustainability Technical University of Denmark Kongens Lyngby Denmark"
},
{
"name": "UNION Therapeutics Research Services Hellerup Denmark"
}
],
"authenticated-orcid": false,
"family": "Weiss",
"given": "Anne",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1659-7644",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Goodman",
"given": "James",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1292-7642",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Fisk",
"given": "Marie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2827-214X",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Kulkarni",
"given": "Spoorthy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2336-6550",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Lu",
"given": "Ing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"family": "Gray",
"given": "Joanna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7438-5156",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
},
{
"name": "Cambridge Clinical Trials Unit Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Smith",
"given": "Rona",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4005-5674",
"affiliation": [
{
"name": "Novo Nordisk Foundation Center for Biosustainability Technical University of Denmark Kongens Lyngby Denmark"
},
{
"name": "UNION Therapeutics Hellerup Denmark"
}
],
"authenticated-orcid": false,
"family": "Sommer",
"given": "Morten",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6921-1592",
"affiliation": [
{
"name": "Department of Medicine Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
},
{
"name": "Cambridge Clinical Trials Unit Cambridge University Hospitals NHS Foundation Trust Cambridge UK"
}
],
"authenticated-orcid": false,
"family": "Cheriyan",
"given": "Joseph",
"sequence": "additional"
}
],
"container-title": "British Journal of Pharmacology",
"container-title-short": "British J Pharmacology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bpspubs.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T11:34:34Z",
"timestamp": 1648553674000
},
"deposited": {
"date-parts": [
[
2023,
8,
19
]
],
"date-time": "2023-08-19T15:03:08Z",
"timestamp": 1692457388000
},
"funder": [
{
"DOI": "10.13039/501100000329",
"award": [
"NNF20CC0035580"
],
"doi-asserted-by": "publisher",
"name": "Novo Nordisk UK Research Foundation"
},
{
"DOI": "10.13039/100012774",
"award": [
"0153‐00209"
],
"doi-asserted-by": "publisher",
"name": "Innovationsfonden"
},
{
"DOI": "10.13039/100004325",
"doi-asserted-by": "publisher",
"name": "AstraZeneca"
},
{
"DOI": "10.13039/501100000291",
"doi-asserted-by": "publisher",
"name": "Kidney Research UK"
},
{
"DOI": "10.13039/501100002927",
"doi-asserted-by": "publisher",
"name": "Addenbrooke's Charitable Trust, Cambridge University Hospitals"
},
{
"DOI": "10.13039/100012357",
"doi-asserted-by": "publisher",
"name": "LifeArc"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T17:52:08Z",
"timestamp": 1703699528116
},
"is-referenced-by-count": 23,
"issue": "13",
"issued": {
"date-parts": [
[
2022,
4,
11
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2022,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
11
]
],
"date-time": "2022-04-11T00:00:00Z",
"timestamp": 1649635200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/bph.15843",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/bph.15843",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/bph.15843",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3250-3267",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
4,
11
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.amsu.2021.102779",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_2_1"
},
{
"DOI": "10.1111/bph.15538",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_3_1"
},
{
"DOI": "10.1111/bph.15541",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_4_1"
},
{
"DOI": "10.1111/bph.15542",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_5_1"
},
{
"DOI": "10.5606/ArchRheumatol.2019.7100",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_6_1"
},
{
"DOI": "10.1007/s40519-017-0373-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_7_1"
},
{
"DOI": "10.1007/s10067-018-4164-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_8_1"
},
{
"DOI": "10.1016/s0099-5428(05)32002-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_9_1"
},
{
"DOI": "10.1016/0163-7258(82)90064-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_10_1"
},
{
"DOI": "10.18632/oncotarget.13466",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_11_1"
},
{
"DOI": "10.1016/j.ygyno.2014.04.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_12_1"
},
{
"DOI": "10.1038/nrd1468",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_13_1"
},
{
"DOI": "10.1016/j.lanepe.2021.100084",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_14_1"
},
{
"DOI": "10.1002/cbic.201700500",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_15_1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_16_1"
},
{
"DOI": "10.1016/j.lfs.2018.12.061",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_17_1"
},
{
"DOI": "10.1016/j.ejphar.2020.173438",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_18_1"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_19_1"
},
{
"DOI": "10.1038/nrd.2018.92",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_20_1"
},
{
"DOI": "10.1371/journal.pone.0246803",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_21_1"
},
{
"DOI": "10.1186/s12885-018-4197-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_22_1"
},
{
"DOI": "10.1200/JCO.2018.36.15_suppl.e14536",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_23_1"
},
{
"DOI": "10.1172/jci.insight.128414",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_24_1"
},
{
"DOI": "10.1242/bio.031807",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_25_1"
},
{
"DOI": "10.3390/ijms22157852",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_26_1"
},
{
"DOI": "10.1136/bmj.m3862",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_27_1"
},
{
"article-title": "Pharmacokinetics of anti‐SARS‐CoV agent niclosamide and its analogs in rats",
"author": "Chang Y.‐W.",
"first-page": "329",
"issue": "4",
"journal-title": "Journal of Food and Drug Analysis",
"key": "e_1_2_15_28_1",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1053/j.gastro.2017.02.039",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_29_1"
},
{
"DOI": "10.1021/ml3003082",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_30_1"
},
{
"DOI": "10.1002/jmv.25681",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_31_1"
},
{
"DOI": "10.1371/journal.pone.0184324",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_32_1"
},
{
"DOI": "10.1016/j.jchromb.2021.122862",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_33_1"
},
{
"DOI": "10.1038/srep40159",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_34_1"
},
{
"DOI": "10.1515/znb-1961-0804",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_35_1"
},
{
"key": "e_1_2_15_36_1",
"unstructured": "England N. (2021).COVID treatment developed in the NHS saves a million lives. Retrieved fromhttps://www.england.nhs.uk/2021/03/covid-treatment-developed-in-the-nhs-saves-a-million-lives/"
},
{
"DOI": "10.1016/j.tube.2019.04.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_37_1"
},
{
"DOI": "10.1007/978-1-4939-2438-7_1",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_38_1"
},
{
"DOI": "10.1016/j.pupt.2021.102035",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_39_1"
},
{
"DOI": "10.1038/s41385-020-00359-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_40_1"
},
{
"DOI": "10.1007/s11684-016-0466-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_41_1"
},
{
"DOI": "10.1101/2021.07.11.451951",
"doi-asserted-by": "crossref",
"key": "e_1_2_15_42_1",
"unstructured": "Garrett T. J. Coatsworth H. Mahmud I. Hamerly T. Stephenson C. J. Yazd H. S. Ayers J. Miller M. R. Lednicky J. A. Dinglasan R. R. &Dinglasan R. R.(2021).Niclosamide reverses SARS‐CoV‐2 control of lipophagy.bioRxiv 2021.2007.2011.451951.https://doi.org/10.1101/2021.07.11.451951"
},
{
"DOI": "10.1038/s41467-019-13659-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_43_1"
},
{
"DOI": "10.1038/s41467-021-24007-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_44_1"
},
{
"DOI": "10.3389/fcimb.2020.589505",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_45_1"
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_46_1"
},
{
"DOI": "10.1002/path.4454",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_47_1"
},
{
"DOI": "10.1016/s0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_48_1"
},
{
"DOI": "10.1101/2021.11.03.21265533",
"doi-asserted-by": "crossref",
"key": "e_1_2_15_49_1",
"unstructured": "Gupta A. Gonzalez‐Rojas Y. Juarez E. Casal M. C. Moya J. Falci D. R. Sarkis E. Solis J. Zheng H. Scott N. Cathcart A. L. Parra S. Sager J. E. Austin D. Peppercorn A. Alexander E. Yeh W. W. Brinson C. Aldinger M. &Shapiro A. E.(2021).Effect of the neutralizing SARS‐CoV‐2 antibody sotrovimab in preventing progression of COVID‐19: A randomized clinical trial.medRxiv 2021.2011.2003.21265533.https://doi.org/10.1101/2021.11.03.21265533"
},
{
"DOI": "10.1088/1748-605X/aa7105",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_50_1"
},
{
"DOI": "10.1038/s41598-019-47707-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_51_1"
},
{
"DOI": "10.3389/fphar.2017.00110",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_52_1"
},
{
"DOI": "10.3389/fphar.2018.01544",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_53_1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_54_1"
},
{
"DOI": "10.1074/jbc.M508381200",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_55_1"
},
{
"DOI": "10.1038/clpt.2013.1",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_56_1"
},
{
"DOI": "10.1128/aac.01952-12",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_57_1"
},
{
"DOI": "10.1186/s12967-021-02760-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_58_1"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_59_1"
},
{
"DOI": "10.1128/aac.00819-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_60_1"
},
{
"DOI": "10.1158/0008-5472.Can-09-3950",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_61_1"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_62_1"
},
{
"DOI": "10.1038/s41598-019-45095-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_63_1"
},
{
"DOI": "10.1371/journal.ppat.1002976",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_64_1"
},
{
"DOI": "10.1186/1743-422x-7-264",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_65_1"
},
{
"DOI": "10.1371/journal.pntd.0006715",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_66_1"
},
{
"DOI": "10.1126/science.abb5793",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_67_1"
},
{
"DOI": "10.1371/journal.pone.0074670",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_68_1"
},
{
"DOI": "10.3892/or.2017.6146",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_69_1"
},
{
"DOI": "10.1038/cr.2017.88",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_70_1"
},
{
"DOI": "10.1158/1535-7163.Mct-16-0912",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_71_1"
},
{
"DOI": "10.1002/pros.23015",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_72_1"
},
{
"DOI": "10.1186/s13063-020-04618-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_73_1"
},
{
"DOI": "10.1186/s40425-019-0733-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_74_1"
},
{
"DOI": "10.1021/acsinfecdis.5b00030",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_75_1"
},
{
"DOI": "10.3390/v11020176",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_76_1"
},
{
"DOI": "10.1038/s41591-020-0952-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_77_1"
},
{
"DOI": "10.3389/fphar.2019.00051",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_78_1"
},
{
"DOI": "10.1016/j.ijantimicag.2018.02.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_79_1"
},
{
"DOI": "10.1016/j.jss.2016.03.051",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_80_1"
},
{
"DOI": "10.4049/jimmunol.1502482",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_81_1"
},
{
"DOI": "10.1038/534314a",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_82_1"
},
{
"DOI": "10.1038/s41598-021-85969-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_83_1"
},
{
"DOI": "10.1001/jama.2020.0757",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_84_1"
},
{
"DOI": "10.7326/0003-4819-102-4-550",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_85_1"
},
{
"DOI": "10.1095/biolreprod.116.140236",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_86_1"
},
{
"DOI": "10.1038/nrd.2018.168",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_87_1"
},
{
"DOI": "10.1371/journal.pone.0124595",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_88_1"
},
{
"DOI": "10.1038/s41598-021-02321-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_89_1"
},
{
"DOI": "10.1371/journal.pone.0198389",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_90_1"
},
{
"DOI": "10.1371/journal.pone.0202709",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_91_1"
},
{
"DOI": "10.1093/biolre/ioaa010",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_92_1"
},
{
"DOI": "10.3390/cells8101261",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_93_1"
},
{
"DOI": "10.7150/jca.41418",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_94_1"
},
{
"DOI": "10.1073/pnas.0505577102",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_95_1"
},
{
"DOI": "10.1101/2021.11.05.21265656",
"doi-asserted-by": "crossref",
"key": "e_1_2_15_96_1",
"unstructured": "Somersan‐Karakaya S. Mylonakis E. Menon V. P. Wells J. C. Ali S. Sivapalasingam S. Sun Y. Bhore R. Mei J. Miller J. Cupelli L. Hooper A. T. Hamilton J. D. Pan C. Pham V. Zhao Y. Hosain R. Mahmood A. Davis J. D. …COVID‐19 Phase 2/3 Hospitalized Trial Team. (2021).REGEN‐COV® for treatment of hospitalized patients with Covid‐19.medRxiv 2021.2011.2005.21265656.https://doi.org/10.1101/2021.11.05.21265656"
},
{
"DOI": "10.18632/oncotarget.8969",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_97_1"
},
{
"DOI": "10.3892/ijmm.2016.2689",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_98_1"
},
{
"DOI": "10.1054/tuld.1999.0212",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_99_1"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_100_1"
},
{
"key": "e_1_2_15_101_1",
"unstructured": "TACTIC‐E Trial. (2020). Retrieved fromhttps://cctu.org.uk/portfolio/COVID-19/TACTIC/TACTIC-E"
},
{
"DOI": "10.1038/s41467-018-07705-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_102_1"
},
{
"DOI": "10.1038/nm.3699",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_103_1"
},
{
"DOI": "10.1038/s41598-018-22037-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_104_1"
},
{
"DOI": "10.3892/ol.2015.3789",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_105_1"
},
{
"DOI": "10.2147/ott.s50065",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_106_1"
},
{
"DOI": "10.1128/aac.00377-16",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_107_1"
},
{
"key": "e_1_2_15_108_1",
"unstructured": "UNION therapeutics. (2020).Niclosamide formulations for treating disease. Retrieved fromhttps://patents.justia.com/patent/11045434"
},
{
"DOI": "10.1016/S2213-2600(21)00270-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_109_1"
},
{
"DOI": "10.7150/jca.26948",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_110_1"
},
{
"DOI": "10.1042/bcj20180385",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_111_1"
},
{
"DOI": "10.1038/s41598-018-30692-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_112_1"
},
{
"DOI": "10.1016/j.antiviral.2016.10.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_113_1"
},
{
"DOI": "10.1038/2211016a0",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_114_1"
},
{
"DOI": "10.1371/journal.pone.0260958",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_115_1"
},
{
"DOI": "10.1021/jm070295s",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_116_1"
},
{
"DOI": "10.1158/1078-0432.Ccr-12-2895",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_117_1"
},
{
"key": "e_1_2_15_118_1",
"unstructured": "World Health Organization. (2022).WHO coronavirus (COVID‐19) dashboard. Retrieved fromhttps://covid19.who.int/"
},
{
"DOI": "10.1128/aac.48.7.2693-2696.2004",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_119_1"
},
{
"DOI": "10.1016/j.lfs.2020.118522",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_120_1"
},
{
"DOI": "10.1016/j.bj.2020.05.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_121_1"
},
{
"DOI": "10.1371/journal.pone.0085887",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_122_1"
},
{
"DOI": "10.1016/j.lfs.2020.118458",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_123_1"
},
{
"DOI": "10.1172/JCI65364",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_124_1"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_125_1"
},
{
"DOI": "10.3892/mmr.2017.8301",
"doi-asserted-by": "publisher",
"key": "e_1_2_15_126_1"
}
],
"reference-count": 125,
"references-count": 125,
"relation": {},
"resource": {
"primary": {
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15843"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Niclosamide—A promising treatment for COVID‐19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "179"
}