Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2100433, REMAP-CAP, NCT02735707, Apr 2021
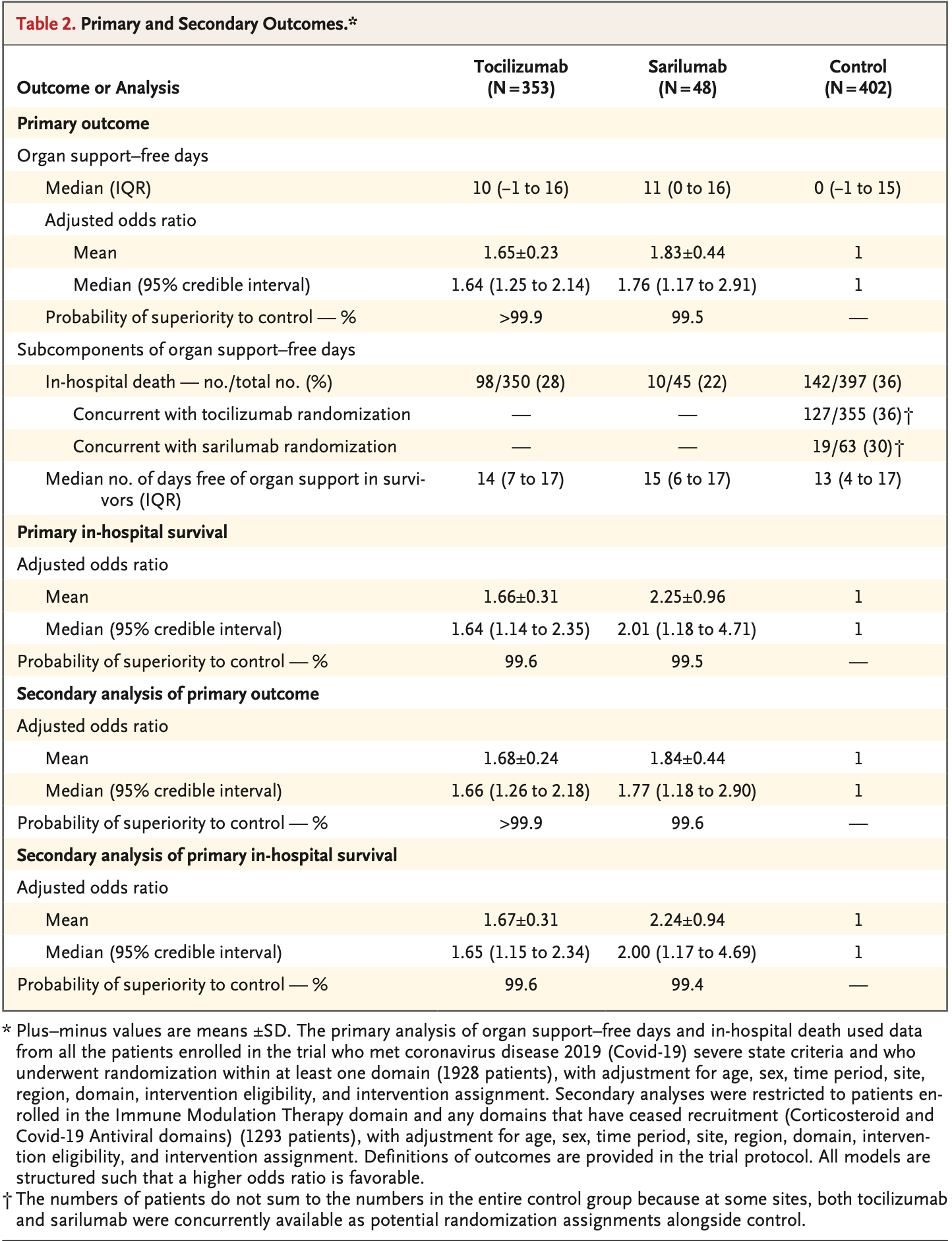
RCT 803 critically ill COVID-19 patients showing improved outcomes with tocilizumab and sarilumab. There was only 48 sarilumab patients and the model used shrinks the posterior distribution for each intervention effect toward the overall estimate for the combined drugs. The concurrent event counts for sarilumab may be more accurate.
Study covers sarilumab and tocilizumab.
|
risk of death, 26.3% lower, RR 0.74, p = 0.39, treatment 10 of 45 (22.2%), control 19 of 63 (30.2%), NNT 13, concurrent control patients.
|
|
risk of death, 50.2% lower, HR 0.50, p = 0.048, treatment 45, control 397, inverted to make HR<1 favor treatment, including non-concurrent control patients.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gordon et al., 22 Apr 2021, Randomized Controlled Trial, multiple countries, peer-reviewed, 62 authors, trial NCT02735707 (history) (REMAP-CAP).
Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2100433
19) is unclear.
Appendix The members of the writing committee are as follows: Prof.
References
Angus, Berry, Lewis, The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design, Ann Am Thorac Soc
Angus, Derde, Al-Beidh, Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial, JAMA
Fajgenbaum, June, Cytokine storm, N Engl J Med
Gremese, Cingolani, Bosello, Sarilumab use in severe SARS-CoV-2 pneumonia, EClinicalMedicine
Hermine, Mariette, Tharaux, Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial, JAMA Intern Med
Laterre, Berry, Blemings, Effect of selepressin vs placebo on ventilator-and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial, JAMA
Pairo-Castineira, Clohisey, Klaric, Genetic mechanisms of critical illness in Covid-19
Rosas, Bräu, Waters, To-cilizumab in hospitalized patients with severe Covid-19 pneumonia, N Engl J Med
Salama, Han, Yau, Tocilizu mab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Salvarani, Dolci, Massari, Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial, JAMA Intern Med
Sinha, Calfee, Cherian, Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study, Lancet Respir Med
Somers, Eschenauer, Troost, Tocilizumab for treatment of mechanically ventilated patients with COVID-19, Clin Infect Dis
Stone, Frigault, Nj, Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
The, Group, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Veiga, Prats, Farias, Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial, BMJ
Viele, Berry, Neuenschwander, Use of historical control data for assessing treatment effects in clinical trials, Pharm Stat
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A
Zhou, Fu, Zheng, Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients, Natl Sci Rev
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zhu, Pang, Ji, Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis, J Med Virol
DOI record:
{
"DOI": "10.1056/nejmoa2100433",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2100433",
"alternative-id": [
"10.1056/NEJMoa2100433"
],
"author": [
{
"affiliation": [],
"name": "The REMAP-CAP Investigators",
"sequence": "first"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
25
]
],
"date-time": "2021-02-25T22:01:17Z",
"timestamp": 1614290477000
},
"deposited": {
"date-parts": [
[
2021,
4,
21
]
],
"date-time": "2021-04-21T21:07:44Z",
"timestamp": 1619039264000
},
"funder": [
{
"DOI": "10.13039/100010270",
"award": [
"215522"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010270",
"id-type": "DOI"
}
],
"name": "Wellcome Trust Innovations Project"
},
{
"DOI": "10.1039/501100000272",
"award": [
"RP-2015-06-18"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.1039/501100000272",
"id-type": "DOI"
}
],
"name": "National Institute for Health Research, Research Professorship"
},
{
"DOI": "10.1039/501100000272",
"award": [
"CS-2016-16-011"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.1039/501100000272",
"id-type": "DOI"
}
],
"name": "National Institute for Health Research, Clinician ScientistNational Institute for Health Research, Research Professorship"
},
{
"DOI": "10.13039/100011102",
"award": [
"#602525"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100011102",
"id-type": "DOI"
}
],
"name": "European Union, FP7"
},
{
"DOI": "10.13039/501100007601",
"award": [
"#101003589"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100007601",
"id-type": "DOI"
}
],
"name": "European Union Horizon 2020"
},
{
"DOI": "10.13039/501100000925",
"award": [
"#APP1101719"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000925",
"id-type": "DOI"
}
],
"name": "Australian National Health and Medical Research Council"
},
{
"DOI": "10.13039/501100001505",
"award": [
"#16/631"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001505",
"id-type": "DOI"
}
],
"name": "Health Research Council of New Zealand"
},
{
"DOI": "10.13039/501100000024",
"award": [
"#158584"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000024",
"id-type": "DOI"
}
],
"name": "Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program"
},
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "National Institute for Health Research"
},
{
"DOI": "10.13039/100010415",
"award": [
"CTN 2014-012"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100010415",
"id-type": "DOI"
}
],
"name": "Health Research Board of Ireland"
},
{
"DOI": "10.13039/501100005737",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100005737",
"id-type": "DOI"
}
],
"name": "UPMC Learning While Doing Program"
},
{
"DOI": "10.13039/100001006",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100001006",
"id-type": "DOI"
}
],
"name": "Breast Cancer Research Foundation"
},
{
"DOI": "10.13039/501100004571",
"award": [
"PHRC-20-0147"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004571",
"id-type": "DOI"
}
],
"name": "French Ministry of Health"
},
{
"DOI": "10.13039/501100016056",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100016056",
"id-type": "DOI"
}
],
"name": "Minderoo Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
23
]
],
"date-time": "2024-09-23T04:22:51Z",
"timestamp": 1727065371588
},
"is-referenced-by-count": 1326,
"issue": "16",
"issued": {
"date-parts": [
[
2021,
4,
22
]
]
},
"journal-issue": {
"issue": "16",
"published-print": {
"date-parts": [
[
2021,
4,
22
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
22
]
],
"date-time": "2021-04-22T00:00:00Z",
"timestamp": 1619049600000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2100433",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "1491-1502",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
4,
22
]
]
},
"published-print": {
"date-parts": [
[
2021,
4,
22
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1016/j.eclinm.2020.100553",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1093/cid/ciaa954",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1001/jamainternmed.2020.6615",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1056/NEJMoa2030340",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1056/NEJMoa2028700",
"author": "Rosas IO",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N Engl J Med",
"key": "r10",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n84",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1513/AnnalsATS.202003-192SD",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1001/jama.2020.17022",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1001/jama.2019.14607",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1002/pst.1589",
"doi-asserted-by": "publisher",
"key": "r15"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "r17"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "r18"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "r19"
},
{
"DOI": "10.1002/jmv.26085",
"doi-asserted-by": "publisher",
"key": "r21"
},
{
"DOI": "10.1038/s41586-020-03065-y",
"doi-asserted-by": "publisher",
"key": "r22"
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "r25"
},
{
"DOI": "10.1016/S2213-2600(20)30366-0",
"doi-asserted-by": "publisher",
"key": "r26"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2100433"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19",
"type": "journal-article",
"volume": "384"
}