SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication
et al., Journal of Clinical Medicine, doi:10.3390/jcm12186079, Sep 2023
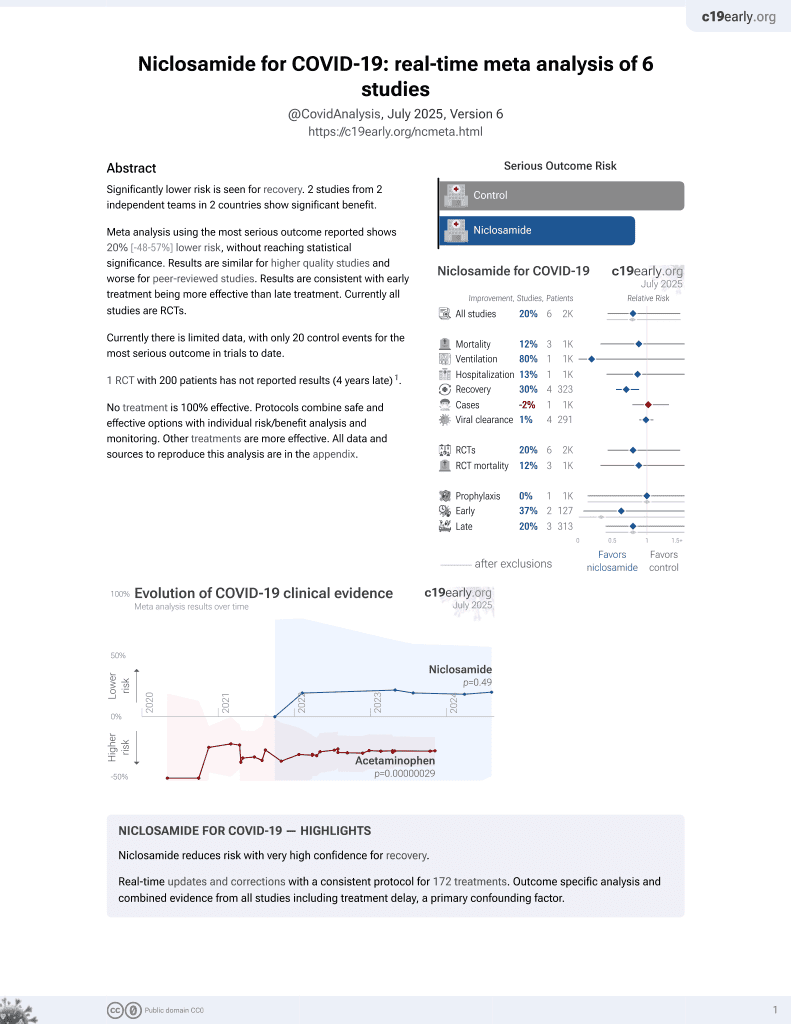
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
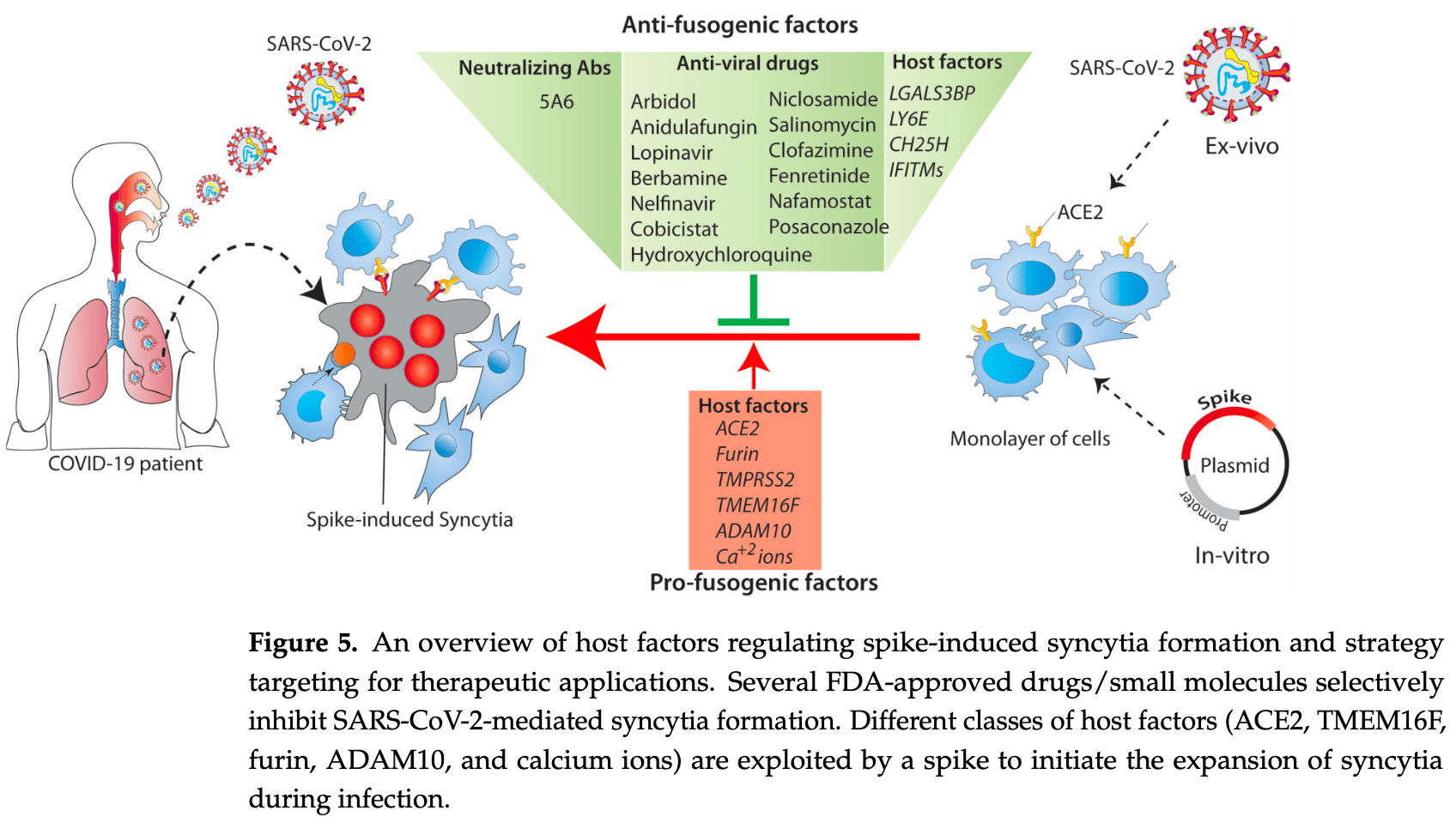
Review of the molecular mechanisms of SARS-CoV-2 spike-induced syncytia formation and potential anti-fusogenic therapeutic strategies. The SARS-CoV-2 spike protein interacts with the ACE2 receptor on adjacent cells, triggering abnormal fusion and formation of syncytia which are beneficial for viral replication, transmission, and immune evasion, contributing to COVID-19 progression. Authors highlight the involvement of various host factors including ACE2, TMEM16F, furin, ADAM10, and calcium ions in modulating the fusogenic properties of the spike protein. Several FDA-approved drugs and small molecules are discussed that selectively inhibit SARS-CoV-2 spike-mediated syncytia formation, including niclosamide and hydroxychloroquine, showing promise as potential therapeutic strategies to limit viral replication. The highly conserved HR1 and HR2 domains of the spike protein are also attractive targets for developing broad-spectrum viral entry/fusion inhibitors.
Review covers niclosamide and HCQ.
1.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
2.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
3.
Ali et al., SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication, Journal of Clinical Medicine, doi:10.3390/jcm12186079.
4.
Vibhute et al., Niclosamide: a potential treatment option for COVID-19, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850.
5.
Abed et al., Evaluation the plausibility of repurpose of levamisole and niclosamide in treatment of covid-19, Journal of Pharmaceutical Technolgy, doi:10.37662/jpt.2022.999481.
6.
Cesar-Silva et al., The Endolysosomal System: The Acid Test for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23094576.
Ali et al., 20 Sep 2023, peer-reviewed, 3 authors.
Contact: ha504@cam.ac.uk (corresponding author).
SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication
Journal of Clinical Medicine, doi:10.3390/jcm12186079
SARS-CoV-2 infection induces non-physiological syncytia when its spike fusogenic protein on the surface of the host cells interacts with the ACE2 receptor on adjacent cells. Spike-induced syncytia are beneficial for virus replication, transmission, and immune evasion, and contribute to the progression of COVID-19. In this review, we highlight the properties of viral fusion proteins, mainly the SARS-CoV-2 spike, and the involvement of the host factors in the fusion process. We also highlight the possible use of anti-fusogenic factors as an antiviral for the development of therapeutics against newly emerging SARS-CoV-2 variants and how the fusogenic property of the spike could be exploited for biomedical applications.
Author Contributions: H.A.: writing-original draft preparation, and illustration of images; A.N. and Z.I.S.: review and manuscript editing. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
Abbreviations COVID-19: Coronavirus disease 2019, SARS-CoV: Severe acute respiratory syndrome coronavirus, MERS-CoV: Middle East respiratory syndrome coronavirus, ACE2: Angiotensin-converting enzyme 2, DPP4: transmembrane dipeptidyl peptidase 4, HA: hemagglutinin, gp41: glycoprotein 41, HIV-1: human immunodeficiency virus 1, VSVG: vesicular stomatitis virus glycoprotein, gB: glycoprotein B, FAST: fusion-associated small transmembrane, TM: transmembrane, ER: endoplasmic reticulum, RBD: receptor-binding domain, FP: fusion peptide, 6-HB: 6-helical bundle, HR heptapeptide repeat, VOCs: variants of concern, WHO: world health organisation, COPI: coatomer complex I, TMPRSS2: Transmembrane Serine Protease 2, ADAM: disintegrin and metalloprotease, SADS-CoV: swine acute diarrhoea syndrome coronavirus, IFITMs: Interferon-induced transmembrane proteins, ZMPSTE24: Zinc metallopeptidase STE24, CH25H: Cholesterol 25-hydroxylase, MHV-68: Murine gammaherpesvirus-68, LY6E: Lymphocyte antigen 6E, Gal-3BP: Galectin-3binding protein, SERCA: sarcoendoplamic reticulum Ca 2+ ATPase, HCQ: hydroxychloroquine, CYP3As: cytochrome P450-3As, CHIKV: Chikungunya virus,..
References
Abrams, Johnson, Perelman, Zhang, Endapally et al., Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol, Nat. Microbiol, doi:10.1038/s41564-020-0701-5
Ahamad, Ali, Secco, Giacca, Gupta, Anti-Fungal Drug Anidulafungin Inhibits SARS-CoV-2 Spike-Induced Syncytia Formation by Targeting ACE2-Spike Protein Interaction, Front. Genet, doi:10.3389/fgene.2022.866474
Ali, Braga, Giacca, Cardiac regeneration and remodelling of the cardiomyocyte cytoarchitecture, FEBS J, doi:10.1111/febs.15146
Amini-Bavil-Olyaee, Choi, Lee, Shi, Huang et al., The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry, Cell Host Microbe, doi:10.1016/j.chom.2013.03.006
Appay, Sauce, Immune activation and inflammation in HIV-1 infection: Causes and consequences, J. Pathol, doi:10.1002/path.2276
Arribas, Esselens, ADAM17 as a therapeutic target in multiple diseases, Curr. Pharm. Des, doi:10.2174/138161209788682398
Artini, Natoli, Tinari, Costanzo, Marinelli et al., Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients, J. Hepatol, doi:10.1016/S0168-8278(96)80076-6
Asarnow, Wang, Lee, Hu, Huang et al., Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia, Cell, doi:10.1016/j.cell.2021.04.033
Avraham, Melamed, Achdout, Erez, Israeli et al., Antiviral activity of glucosylceramide synthase inhibitors in alphavirus infection of the central nervous system, Brain Commun, doi:10.1093/braincomms/fcad086
Azab, Gramatica, Herrmann, Osterrieder, Binding of alphaherpesvirus glycoprotein H to surface alpha4beta1integrins activates calcium-signaling pathways and induces phosphatidylserine exposure on the plasma membrane, mBio, doi:10.1128/mBio.01552-15
Backovic, Jardetzky, Class III viral membrane fusion proteins, Curr. Opin. Struct. Biol, doi:10.1016/j.sbi.2009.02.012
Bailey, Zhong, Huang, Farzan, IFITM-Family Proteins: The Cell's First Line of Antiviral Defense, Annu. Rev. Virol, doi:10.1146/annurev-virology-031413-085537
Barrett, Dutch, Viral Membrane Fusion and the Transmembrane Domain, Viruses, doi:10.3390/v12070693
Benton, Wrobel, Xu, Roustan, Martin et al., Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion, Nature, doi:10.1038/s41586-020-2772-0
Bertram, Dijkman, Habjan, Heurich, Gierer et al., TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium, J. Virol, doi:10.1128/JVI.03372-12
Boehm, Kronig, Neher, Eckerle, Vetter et al., Geneva Centre for Emerging Viral Diseases. Novel SARS-CoV-2 variants: The pandemics within the pandemic, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.05.022
Bolze, Mommert, Mallet, Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies, Prog. Mol. Biol. Transl. Sci, doi:10.1016/bs.pmbts.2016.12.005
Bosmuller, Matter, Fend, Tzankov, The pulmonary pathology of COVID-19, Virchows. Arch, doi:10.1007/s00428-021-03053-1
Boson, Legros, Zhou, Siret, Mathieu et al., The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles, J. Biol. Chem, doi:10.1074/jbc.RA120.016175
Braga, Ali, Secco, Chiavacci, Neves et al., Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature, doi:10.1038/s41586-021-03491-6
Braun, Sauter, Furin-mediated protein processing in infectious diseases and cancer, Clin. Transl. Immunol, doi:10.1002/cti2.1073
Brice, Diamond, Antiviral Activities of Human Host Defense Peptides, Curr. Med. Chem, doi:10.2174/0929867326666190805151654
Brukman, Uygur, Podbilewicz, Chernomordik, How cells fuse, J. Cell Biol, doi:10.1083/jcb.201901017
Buchrieser, Dufloo, Hubert, Monel, Planas et al., Syncytia formation by SARS-CoV-2-infected cells, EMBO J, doi:10.15252/embj.2020106267
Bullough, Hughson, Skehel, Wiley, Structure of influenza haemagglutinin at the pH of membrane fusion, Nature, doi:10.1038/371037a0
Bussani, Schneider, Zentilin, Collesi, Ali et al., Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology, EBioMedicine, doi:10.1016/j.ebiom.2020.103104
Calabrese, Sures, Pompetti, Natoli, Palka et al., The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25, Cytogenet. Cell Genet, doi:10.1159/000133969
Callahan, Popernack, Tsutsui, Truong, Schlegel et al., Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells, J. Immunol, doi:10.4049/jimmunol.170.9.4840
Callaway, Fast-spreading COVID variant can elude immune responses, Nature, doi:10.1038/d41586-021-00121-z
Cappelletto, Allan, Crescente, Schneider, Bussani et al., SARS-CoV-2 Spike protein activates TMEM16F-mediated platelet procoagulant activity, Front. Cardiovasc. Med, doi:10.3389/fcvm.2022.1013262
Casasampere, Ordonez, Pou, Casas, Inhibitors of dihydroceramide desaturase 1: Therapeutic agents and pharmacological tools to decipher the role of dihydroceramides in cell biology, Chem. Phys. Lipids, doi:10.1016/j.chemphyslip.2015.07.025
Cattin-Ortola, Welch, Maslen, Papa, James et al., Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation, Nat. Commun, doi:10.1038/s41467-021-25589-1
Chan, Arthur, Morstein, Jin, Bhat et al., Evolutionarily related small viral fusogens hijack distinct but modular actin nucleation pathways to drive cell-cell fusion, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2007526118
Chan, Son, Schmid, Fletcher, A viral fusogen hijacks the actin cytoskeleton to drive cell-cell fusion, Elife, doi:10.7554/eLife.51358
Chen, Cao, Zhong, Host Calcium Channels and Pumps in Viral Infections, Cells, doi:10.3390/cells9010094
Chen, Skehel, Wiley, N-and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil, Proc. Natl. Acad. Sci, doi:10.1073/pnas.96.16.8967
Cheng, Chao, Li, Chiu, Kao et al., Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects, Cell. Rep, doi:10.1016/j.celrep.2020.108254
Cheng, Chao, Li, Wang, Kao et al., D614G Substitution of SARS-CoV-2 Spike Protein Increases Syncytium Formation and Virus Titer via Enhanced Furin-Mediated Spike Cleavage, mBio, doi:10.1128/mBio.00587-21
Ciechonska, Duncan, Reovirus FAST proteins: Virus-encoded cellular fusogens, Trends Microbiol, doi:10.1016/j.tim.2014.08.005
Clemens, Ye, Zhou, Kim, Pease et al., SARS-CoV-2 spike protein-mediated cardiomyocyte fusion may contribute to increased arrhythmic risk in COVID-19, PLoS ONE, doi:10.1371/journal.pone.0282151
Cohen, Melikyan, Implications of a fusion peptide structure, Nat. Struct. Biol, doi:10.1038/90341
Corti, Purcell, Snell, Veesler, Tackling COVID-19 with neutralizing monoclonal antibodies, Cell, doi:10.1016/j.cell.2021.05.005
Coutard, Valle, De Lamballerie, Canard, Seidah et al., The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade, Antiviral Res, doi:10.1016/j.antiviral.2020.104742
Cowley, Fuller, Rand, Parsegian, Measurement of repulsive forces between charged phospholipid bilayers, Biochemistry, doi:10.1021/bi00608a034
Das, Bulow, Diehl, Durham, Senjobe et al., Conformational changes in the Ebola virus membrane fusion machine induced by pH, Ca 2+ , and receptor binding, PLoS Biol, doi:10.1371/journal.pbio.3000626
Desai, Marin, Chin, Savidis, Brass et al., IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion, PLoS Pathog, doi:10.1371/journal.ppat.1004048
Dias, Soares, Ferreira, Sacramento, Fintelman-Rodrigues et al., Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators, PLoS Pathog, doi:10.1371/journal.ppat.1009127
Dittmar, Lee, Whig, Segrist, Li et al., Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-CoV-2, Cell. Rep, doi:10.1016/j.celrep.2021.108959
Drosten, Gunther, Preiser, Van Der Werf, Brodt et al., Identification of a novel coronavirus in patients with severe acute respiratory syndrome, N. Engl. J. Med, doi:10.1056/NEJMoa030747
Dube, Rey, Kielian, Rubella virus: First calcium-requiring viral fusion protein, PLoS Pathog, doi:10.1371/journal.ppat.1004530
Duncan, Fusogenic Reoviruses and Their Fusion-Associated Small Transmembrane (FAST) Proteins, Annu. Rev. Virol, doi:10.1146/annurev-virology-092818-015523
Dyall, Coleman, Hart, Venkataraman, Holbrook et al., Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection, Antimicrob. Agents Chemother, doi:10.1128/AAC.03036-14
Eckert, Kim, Mechanisms of viral membrane fusion and its inhibition, Annu. Rev. Biochem, doi:10.1146/annurev.biochem.70.1.777
Fenwick, Joo, Jacquier, Noto, Banga et al., T-cell exhaustion in HIV infection, Immunol. Rev, doi:10.1111/imr.12823
Filer, Bik, Parsonage, Fitton, Trebilcock et al., Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways, Arthritis Rheum, doi:10.1002/art.24574
Frankel, Wenig, Burke, Mannan, Thompson et al., Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid, Science, doi:10.1126/science.272.5258.115
Friedman, Manly, Mcmahon, Kerr, Stark, Transcriptional and posttranscriptional regulation of interferoninduced gene expression in human cells, Cell, doi:10.1016/0092-8674(84)90270-8
Fu, Wang, Li, Dorf, ZMPSTE24 defends against influenza and other pathogenic viruses, J. Exp. Med, doi:10.1084/jem.20161270
Gall, Bobe, Reiss, Horiuchi, Niu et al., ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha, Mol. Biol. Cell, doi:10.1091/mbc.e08-11-1135
Gallo, Team, Gentile, Antonini, Iacobelli, Increased Gal-3BP plasma levels in hospitalized patients infected with SARS-CoV-2, Clin. Exp. Med, doi:10.1007/s10238-021-00788-8
Gauchotte, Venard, Segondy, Cadoz, Esposito-Fava et al., SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study, Int. J. Legal Med, doi:10.1007/s00414-020-02500-z
Geyer, Arend, Doll, Louiset, Virreira Winter et al., High-resolution serum proteome trajectories in COVID-19 reveal patient-specific seroconversion, EMBO Mol. Med, doi:10.15252/emmm.202114167
Giansanti, Strating, Defourny, Cesonyte, Bottino et al., Dynamic remodelling of the human host cell proteome and phosphoproteome upon enterovirus infection, Nat. Commun, doi:10.1038/s41467-020-18168-3
Gopal, Padayatchi, Metcalfe, O'donnell, Systematic review of clofazimine for the treatment of drug-resistant tuberculosis, Int. J. Tuberc. Lung Dis, doi:10.5588/ijtld.12.0144
Gutmann, Takov, Burnap, Singh, Ali et al., SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care, Nat. Commun, doi:10.1038/s41467-021-23494-1
Harrison, Viral membrane fusion, Nat. Struct. Mol. Biol, doi:10.1038/nsmb.1456
Harrison, Viral membrane fusion, Virology, doi:10.1016/j.virol.2015.03.043
Hay, Calcium: A fundamental regulator of intracellular membrane fusion?, EMBO Rep, doi:10.1038/sj.embor.7400921
Hayashi, Nemoto-Sasaki, Tanikawa, Oka, Tsuchiya et al., Sphingomyelin synthase 2, but not sphingomyelin synthase 1, is involved in HIV-1 envelope-mediated membrane fusion, J. Biol. Chem, doi:10.1074/jbc.M114.574285
Hayashi, Tsuchiya, Yamamoto, Nemoto-Sasaki, Tanigawa et al., N-(4-Hydroxyphenyl) Retinamide Suppresses SARS-CoV-2 Spike Protein-Mediated Cell-Cell Fusion by a Dihydroceramide Delta4-Desaturase 1-Independent Mechanism, J. Virol, doi:10.1128/JVI.00807-21
He, Zhang, Chen, Li, Increased LGALS3 expression independently predicts shorter overall survival in patients with the proneural subtype of glioblastoma, Cancer Med, doi:10.1002/cam4.2075
Helm, Israelachvili, Mcguiggan, Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers, Science, doi:10.1126/science.2814514
Hepojoki, Strandin, Hetzel, Sironen, Klingstrom et al., Acute hantavirus infection induces galectin-3-binding protein, J. Gen. Virol, doi:10.1099/vir.0.066837-0
Herschke, Plumet, Duhen, Azocar, Druelle et al., Cell-cell fusion induced by measles virus amplifies the type I interferon response, J. Virol, doi:10.1128/JVI.00078-07
Heurich, Hofmann-Winkler, Gierer, Liepold, Jahn et al., TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein, J. Virol, doi:10.1128/JVI.02202-13
Hickford, Frankenberg, Shaw, Renfree, Evolution of vertebrate interferon inducible transmembrane proteins, BMC Genom, doi:10.1186/1471-2164-13-155
Hoffmann, Kleine-Weber, Pohlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Mol. Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Houri, Huang, Nalbantoglu, The Coxsackievirus and Adenovirus Receptor (CAR) undergoes ectodomain shedding and regulated intramembrane proteolysis (RIP), PLoS ONE, doi:10.1371/journal.pone.0073296
Huang, Bailey, Weyer, Radoshitzky, Becker et al., Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus, PLoS Pathog, doi:10.1371/journal.ppat.1001258
Huang, Incognito, Cheng, Ulbrandt, Wu, Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion, J. Virol, doi:10.1128/JVI.02699-09
Inohara, Akahani, Koths, Raz, Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion, Cancer Res
Islam, Calcium Signaling: From Basic to Bedside, Adv. Exp. Med. Biol, doi:10.1007/978-3-030-12457-1_1
Izaguirre, The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases, Viruses, doi:10.3390/v11090837
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat. Rev. Mol. Cell. Biol, doi:10.1038/s41580-021-00418-x
Jana, Bhattacharya, Mayilsamy, Banerjee, Bhattacharje et al., Targeting an evolutionarily conserved "E-L-L" motif in the spike protein to develop a small molecule fusion inhibitor against SARS-CoV-2, bioRxiv, doi:10.1101/2022.03.16.484554
Jang, Shin, Yoon, Go, Lee et al., Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function, J. Virol, doi:10.1128/JVI.01441-18
Jangamreddy, Ghavami, Grabarek, Kratz, Wiechec et al., Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: Differences between primary and cancer cells, Biochim. Biophys. Acta, doi:10.1016/j.bbamcr.2013.04.011
Jia, Liu, Tian, Xiong, Xu et al., Potent neutralizing RBD-specific antibody cocktail against SARS-CoV-2 and its mutant, MedComm, doi:10.1002/mco2.79
Jiang, Li, Qaed, Zhang, Song et al., Salinomycin, as an autophagy modulator--a new avenue to anticancer: A review, J. Exp. Clin. Cancer Res, doi:10.1186/s13046-018-0680-z
Jocher, Grass, Tschirner, Riepler, Breimann et al., ADAM10 and ADAM17 promote SARS-CoV-2 cell entry and spike protein-mediated lung cell fusion, EMBO Rep, doi:10.15252/embr.202154305
Johnson, Gonzales, Olson, Wright, Graham, The histopathology of fatal untreated human respiratory syncytial virus infection, Mod. Pathol, doi:10.1038/modpathol.3800725
Johnson, Xie, Bailey, Kalveram, Lokugamage et al., Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis, Nature, doi:10.1038/s41586-021-03237-4
Kanai, Kawagishi, Sakai, Nouda, Shimojima et al., Cell-cell fusion induced by reovirus FAST proteins enhances replication and pathogenicity of non-enveloped dsRNA viruses, PLoS Pathog, doi:10.1371/journal.ppat.1007675
Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes, Cell, doi:10.1016/j.cell.2020.11.025
Kielian, Rey, Virus membrane-fusion proteins: More than one way to make a hairpin, Nat. Rev. Microbiol, doi:10.1038/nrmicro1326
Kim, Yoon, Park, Furin cleavage is required for swine acute diarrhea syndrome coronavirus spike protein-mediated cell-cell fusion, Emerg. Microbes Infect, doi:10.1080/22221751.2022.2114850
Ko, Chang, Byun, Ianevski, Choi et al., Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19, Viruses, doi:10.3390/v13040651
Koch, Manzur, Shan, Structure-based models of cadherin-mediated cell adhesion: The evolution continues, Cell. Mol. Life Sci, doi:10.1007/s00018-004-4006-2
Koot, Keet, Vos, De Goede, Roos et al., Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS, Ann. Intern. Med, doi:10.7326/0003-4819-118-9-199305010-00004
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus, Cell, doi:10.1016/j.cell.2020.06.043
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat. Med, doi:10.1038/nm1267
Kusnierz-Cabala, Maziarz, Dumnicka, Dembinski, Kapusta et al., Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study, Biomolecules, doi:10.3390/biom11081136
Lai, Millet, Daniel, Freed, Whittaker, The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner, J. Mol. Biol, doi:10.1016/j.jmb.2017.10.017
Lambert, Yarski, Warner, Thornhill, Parkin et al., Tumor necrosis factoralpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2), J. Biol. Chem, doi:10.1074/jbc.M505111200
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Lau, Luk, Wong, Li, Zhu et al., Possible Bat Origin of Severe Acute Respiratory Syndrome Coronavirus 2, Emerg. Infect. Dis, doi:10.3201/eid2607.200092
Leroy, Han, Woottum, Bracq, Bouchet et al., Virus-Mediated Cell-Cell Fusion, Int. J. Mol. Sci, doi:10.3390/ijms21249644
Li, Fu, Wang, Dorf, ZMPSTE24 Is Downstream Effector of Interferon-Induced Transmembrane Antiviral Activity, DNA Cell. Biol, doi:10.1089/dna.2017.3791
Li, Moore, Vasilieva, Sui, Wong et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Li, Sempowski, Saunders, Acharya, Haynes, SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment, Annu. Rev. Med, doi:10.1146/annurev-med-042420-113838
Li, Wu, Nie, Zhang, Hao et al., The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity, Cell, doi:10.1016/j.cell.2020.07.012
Lin, Li, Wang, Shi, Syncytia formation during SARS-CoV-2 lung infection: A disastrous unity to eliminate lymphocytes, Cell. Death Differ, doi:10.1038/s41418-021-00795-y
Liu, Liu, Chen, Lin, Huang et al., Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection, Int. J. Mol. Sci, doi:10.3390/ijms17060832
Liu, Sanchez, Aliyari, Lu, Cheng, Systematic identification of type I and type II interferon-induced antiviral factors, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1114981109
Liu, Wei, Xu, Zhao, Huang et al., SARS-CoV-2 spike protein-induced cell fusion activates the cGAS-STING pathway and the interferon response, Sci. Signal, doi:10.1126/scisignal.abg8744
Liu, Xiao, Chen, He, Niu et al., Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors, Lancet, doi:10.1016/S0140-6736(04)15788-7
Lorizate, Krausslich, Role of lipids in virus replication, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a004820
Lozada, Barlow, Gonzalez, Lubin-Germain, Ballet, Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses, Front. Chem, doi:10.3389/fchem.2021.689006
Lu, Hu, Wang, Qi, Gao et al., Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26, Nature, doi:10.1038/nature12328
Lu, Huang, Yang, Chang, Lee et al., siRNA silencing of angiotensin-converting enzyme 2 reduced severe acute respiratory syndrome-associated coronavirus replications in Vero E6 cells, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-008-0495-5
Lu, Liu, Zhu, Chan, Qin et al., Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor, Nat. Commun, doi:10.1038/ncomms4067
Ma, Buckalew, Du, Kiyoshi, Alford et al., Gap junction coupling confers isopotentiality on astrocyte syncytium, Glia, doi:10.1002/glia.22924
Madu, Roth, Belouzard, Whittaker, Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide, J. Virol, doi:10.1128/JVI.00079-09
Mannar, Saville, Zhu, Srivastava, Berezuk et al., SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein-ACE2 complex, Science, doi:10.1126/science.abn7760
Martinez, Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.00399-20
Mcbride, Li, Machamer, The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein, J. Virol, doi:10.1128/JVI.02146-06
Mcnamara, Smyth, The pathogenesis of respiratory syncytial virus disease in childhood, Br. Med. Bull, doi:10.1093/bmb/61.1.13
Messner, Demichev, Wendisch, Michalick, White et al., Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection, Cell. Syst, doi:10.1016/j.cels.2020.05.012
Mlcochova, Kemp, Dhar, Papa, Meng et al., SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion, Nature, doi:10.1038/s41586-021-03944-y
Morizono, Chen, Role of phosphatidylserine receptors in enveloped virus infection, J. Virol, doi:10.1128/JVI.03287-13
Moss, Sklair-Tavron, Nudelman, Drug insight: Tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis, Nat. Clin. Pract. Rheumatol, doi:10.1038/ncprheum0797
Mou, Xie, Male infertility-related molecules involved in sperm-oocyte fusion, J. Reprod. Dev, doi:10.1262/jrd.2016-108
Mudhasani, Tran, Retterer, Radoshitzky, Kota et al., IFITM-2 and IFITM-3 but not IFITM-1 restrict Rift Valley fever virus, J. Virol, doi:10.1128/JVI.03382-12
Musarrat, Chouljenko, Dahal, Nabi, Chouljenko et al., The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections, J. Med. Virol, doi:10.1002/jmv.25985
Nanbo, Maruyama, Imai, Ujie, Fujioka et al., Ebola virus requires a host scramblase for externalization of phosphatidylserine on the surface of viral particles, PLoS Pathog, doi:10.1371/journal.ppat.1006848
Nathan, Lai, Millet, Straus, Freed et al., Calcium Ions Directly Interact with the Ebola Virus Fusion Peptide To Promote Structure-Function Changes That Enhance Infection, ACS Infect. Dis, doi:10.1021/acsinfecdis.9b00296
Natoli, Iacobelli, Ghinelli, Unusually high level of a tumor-associated antigen in the serum of human immunodeficiency virus-seropositive individuals, J. Infect. Dis, doi:10.1093/infdis/164.3.616
Navaratnarajah, Pease, Halfmann, Taye, Barkhymer et al., Highly Efficient SARS-CoV-2 Infection of Human Cardiomyocytes: Spike Protein-Mediated Cell Fusion and Its Inhibition, J. Virol, doi:10.1128/JVI.01368-21
Nishimura, Shimojima, Tano, Miyamura, Wakita et al., Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71, Nat. Med, doi:10.1038/nm.1961
Osorio, Sfera, Anton, Thomas, Andronescu et al., Virus-Induced Membrane Fusion in Neurodegenerative Disorders, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.845580
Ostergaard, Nielsen, Iversen, Tanassi, Knudsen et al., Unique protein signature of circulating microparticles in systemic lupus erythematosus, Arthritis Rheum, doi:10.1002/art.38065
Peacock, Goldhill, Zhou, Baillon, Frise et al., The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets, Nat. Microbiol, doi:10.1038/s41564-021-00908-w
Pfaender, Mar, Michailidis, Kratzel, Boys et al., LY6E impairs coronavirus fusion and confers immune control of viral disease, Nat. Microbiol, doi:10.1038/s41564-020-0769-y
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Podbilewicz, Virus and cell fusion mechanisms, Annu. Rev. Cell. Dev. Biol, doi:10.1146/annurev-cellbio-101512-122422
Rajah, Hubert, Bishop, Saunders, Robinot et al., SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation, EMBO J, doi:10.15252/embj.2021108944
Rea, Palmieri, Tinari, Natoli, Tagliaferri et al., 90k is a serum marker of poor-prognosis in non-hodgkins-lymphoma patients, Oncol. Rep, doi:10.3892/or.1.4.723
Riva, Yuan, Yin, Martin-Sancho, Matsunaga et al., Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature, doi:10.1038/s41586-020-2577-1
Robson, Khan, Le, Paris, Demirbag et al., Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting, Mol. Cell, doi:10.1016/j.molcel.2020.07.027
Rocheleau, Laroche, Fu, Stewart, Mohamud et al., Identification of a High-Frequency Intrahost SARS-CoV-2 Spike Variant with Enhanced Cytopathic and Fusogenic Effects, mBio, doi:10.1128/mBio.00788-21
Rockx, Kuiken, Herfst, Bestebroer, Lamers et al., Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model, Science, doi:10.1126/science.abb7314
Romi, Gokhman, Wong, Antonovsky, Ludwig et al., ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A, Nat. Commun, doi:10.1038/ncomms5058
Saito, Irie, Suzuki, Maemura, Nasser et al., Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation, Nature, doi:10.1038/s41586-021-04266-9
Sakurai, Kolokoltsov, Chen, Tidwell, Bauta et al., Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment, Science, doi:10.1126/science.1258758
Salsman, Top, Barry, Duncan, A virus-encoded cell-cell fusion machine dependent on surrogate adhesins, PLoS Pathog, doi:10.1371/journal.ppat.1000016
Sampath, Sampath, Millay, Myoblast fusion confusion: The resolution begins, Skelet. Muscle, doi:10.1186/s13395-017-0149-3
Sanders, Jumper, Ackerman, Bracha, Donlic et al., SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation, Elife, doi:10.7554/eLife.65962
Sasaki, Brakebusch, Engel, Timpl, Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin, EMBO J, doi:10.1093/emboj/17.6.1606
Saurav, Tanwar, Ahuja, Motiani, Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance, Mol. Aspects Med, doi:10.1016/j.mam.2021.101004
Schjoldager, Vester-Christensen, Goth, Petersen, Brunak et al., A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing, J. Biol. Chem, doi:10.1074/jbc.M111.287912
Schoggins, Wilson, Panis, Murphy, Jones et al., A diverse range of gene products are effectors of the type I interferon antiviral response, Nature, doi:10.1038/nature09907
Shang, Ye, Shi, Wan, Luo et al., Structural basis of receptor recognition by SARS-CoV-2, Nature, doi:10.1038/s41586-020-2179-y
Shi, Kenney, Kudryashova, Zani, Zhang et al., Opposing activities of IFITM proteins in SARS-CoV-2 infection, EMBO J, doi:10.15252/embj.2020106501
Shilagardi, Spear, Abraham, Griffin, Michaelis, The Integral Membrane Protein ZMPSTE24 Protects Cells from SARS-CoV-2 Spike-Mediated Pseudovirus Infection and Syncytia Formation, mBio, doi:10.1128/mbio.02543-22
Shmulevitz, Epand, Epand, Duncan, Structural and functional properties of an unusual internal fusion peptide in a nonenveloped virus membrane fusion protein, J. Virol, doi:10.1128/JVI.78.6.2808-2818.2004
Shytaj, Fares, Gallucci, Lucic, Tolba et al., The FDA-Approved Drug Cobicistat Synergizes with Remdesivir To Inhibit SARS-CoV-2 Replication In Vitro and Decreases Viral Titers and Disease Progression in Syrian Hamsters, mBio, doi:10.1128/mbio.03705-21
Singh, Mukherji, Basak, Hoffmann, Das, Dynamic Ca(2+) sensitivity stimulates the evolved SARS-CoV-2 spike strain-mediated membrane fusion for enhanced entry, Cell. Rep, doi:10.1016/j.celrep.2022.110694
Singh, Rahman, Ehtesham, Hira, Hasnain, SARS-CoV-2 variants of concern are emerging in India, Nat. Med, doi:10.1038/s41591-021-01397-4
Siripanthong, Nazarian, Muser, Deo, Santangeli et al., Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management, Heart Rhythm, doi:10.1016/j.hrthm.2020.05.001
Starr, Czudnochowski, Liu, Zatta, Park et al., SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape, Nature, doi:10.1038/s41586-021-03807-6
Straus, Bidon, Tang, Jaimes, Whittaker et al., Inhibitors of L-Type Calcium Channels Show Therapeutic Potential for Treating SARS-CoV-2 Infections by Preventing Virus Entry and Spread, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00023
Straus, Tang, Lai, Flegel, Bidon et al., Ca(2+) Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity, J. Virol, doi:10.1128/JVI.00426-20
Suzuki, Yamasoba, Kimura, Wang, Kishimoto et al., Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant, Nature, doi:10.1038/s41586-022-04462-1
Sylwester, Murphy, Shutt, Soll, HIV-induced T cell syncytia are self-perpetuating and the primary cause of T cell death in culture, J. Immunol, doi:10.4049/jimmunol.158.8.3996
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-021-00542-x
Temesgen, Cobicistat, a pharmacoenhancer for HIV treatments, Drugs Today, doi:10.1358/dot.2013.49.4.1947288
Theken, Tang, Sengupta, Fitzgerald, The roles of lipids in SARS-CoV-2 viral replication and the host immune response, J. Lipid Res, doi:10.1016/j.jlr.2021.100129
Thomas, Furin at the cutting edge: From protein traffic to embryogenesis and disease, Nat. Rev. Mol. Cell. Biol, doi:10.1038/nrm934
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: Implications for SARS-CoV-2, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00468-6
Valdebenito, Bessis, Annane, Lorin De La Grandmaison, Cramer-Borde et al., COVID-19 Lung Pathogenesis in SARS-CoV-2 Autopsy Cases, Front. Immunol, doi:10.3389/fimmu.2021.735922
Valle, Kim-Schulze, Huang, Beckmann, Nirenberg et al., An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat. Med, doi:10.1038/s41591-020-1051-9
Verdoodt, Vogt, Schmitz, Liffers, Tannapfel et al., Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species, PLoS ONE, doi:10.1371/journal.pone.0044132
Vitner, Achdout, Avraham, Politi, Cherry et al., Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus, J. Biol. Chem, doi:10.1016/j.jbc.2021.100470
Wall, Wu, Harvey, Kelly, Warchal et al., Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination, Lancet, doi:10.1016/S0140-6736(21)01290-3
Wang, Li, Hui, Tiwari, Zhang et al., Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol, EMBO J, doi:10.15252/embj.2020106057
Wang, Xia, Zhu, Lu, Jiang, Pan-coronavirus fusion inhibitors as the hope for today and tomorrow, Protein Cell, doi:10.1007/s13238-020-00806-7
Wang, Yuan, Zhang, Min, Zhou et al., Impact of cell fusion in myeloma marrow microenvironment on tumor progression, Oncotarget, doi:10.18632/oncotarget.25742
Watanabe, Sakuragi, Noji, Nagata, Single-molecule analysis of phospholipid scrambling by TMEM16F, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1717956115
Weston, Czieso, White, Smith, Kellam et al., A membrane topology model for human interferon inducible transmembrane protein 1, PLoS ONE, doi:10.1371/journal.pone.0104341
Whitlock, Chernomordik, Flagging fusion: Phosphatidylserine signaling in cell-cell fusion, J. Biol. Chem, doi:10.1016/j.jbc.2021.100411
Wrensch, Winkler, Pohlmann, IFITM proteins inhibit entry driven by the MERS-coronavirus spike protein: Evidence for cholesterol-independent mechanisms, Viruses, doi:10.3390/v6093683
Xia, Liu, Wang, Xu, Lan et al., Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion, Cell. Res, doi:10.1038/s41422-020-0305-x
Xia, Yan, Xu, Agrawal, Algaissi et al., A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike, Sci. Adv, doi:10.1126/sciadv.aav4580
Xia, Zhu, Liu, Lan, Xu et al., Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein, Cell. Mol. Immunol, doi:10.1038/s41423-020-0374-2
Xing, Xu, Xu, Liu, Shen et al., A Five-Helix-Based SARS-CoV-2 Fusion Inhibitor Targeting Heptad Repeat 2 Domain against SARS-CoV-2 and Its Variants of Concern, Viruses, doi:10.3390/v14030597
Xu, Shi, Li, Zhou, Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00052
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30076-X
Xu, Wu, Zhang, Manifestations and Mechanism of SARS-CoV2 Mediated Cardiac Injury, Int. J. Biol. Sci, doi:10.7150/ijbs.69677
Yamada, Liu, Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells, J. Virol, doi:10.1128/JVI.00613-09
Yamamoto, Kiso, Sakai-Tagawa, Iwatsuki-Horimoto, Imai et al., The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner, Viruses, doi:10.3390/v12060629
Yamamoto, Matsuyama, Li, Takeda, Kawaguchi et al., Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay, Antimicrob. Agents Chemother, doi:10.1128/AAC.01043-16
Yan, Zhang, Li, Xia, Guo et al., Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2, Science, doi:10.1126/science.abb2762
Yang, Kim, David, Palmer, Jin et al., TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation, Cell, doi:10.1016/j.cell.2012.07.036
Yu, Deng, Zou, Wang, Dai et al., A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses, Nat. Commun, doi:10.1038/ncomms15672
Yuan, Yin, Meng, Chan, Ye et al., Clofazimine broadly inhibits coronaviruses including SARS-CoV-2, Nature, doi:10.1038/s41586-021-03431-4
Zaitseva, Zaitsev, Melikov, Arakelyan, Marin et al., Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine, Cell Host Microbe, doi:10.1016/j.chom.2017.06.012
Zaki, Van Boheemen, Bestebroer, Osterhaus, Fouchier, Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia, N. Engl. J. Med, doi:10.1056/NEJMoa1211721
Zang, Case, Yutuc, Ma, Shen et al., Cholesterol 25hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2012197117
Zani, Yount, Antiviral Protection by IFITM3 In Vivo, Curr. Clin. Microbiol. Rep, doi:10.1007/s40588-018-0103-0
Zeng, Evans, King, Zheng, Oltz et al., SARS-CoV-2 spreads through cell-to-cell transmission, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2111400119
Zhang, Le, Grabau, Mohseni, Kim et al., TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development, Sci. Adv, doi:10.1126/sciadv.aba0310
Zhang, Mann, Syed, Reynolds, Tian et al., Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2109905118
Zhang, Zhang, Zhang, Zhang, Li et al., Berbamine hydrochloride potently inhibits SARS-CoV-2 infection by blocking S protein-mediated membrane fusion, PLoS Negl. Trop. Dis, doi:10.1371/journal.pntd.0010363
Zhang, Zheng, Niu, Zhang, Wang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell. Death Differ, doi:10.1038/s41418-021-00782-3
Zhao, Chen, Li, Chen, Sun, Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities, Viruses, doi:10.3390/v12070727
Zhao, Guo, Liu, Cuconati, Chang et al., Interferon induction of IFITM proteins promotes infection by human coronavirus OC43, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1320856111
Zhao, Meng, Peng, Lam, Zhang et al., Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants, Emerg. Microbes Infect, doi:10.1080/22221751.2022.2051753
Zhao, Sehgal, Hou, Cheng, Shu et al., Identification of Residues Controlling Restriction versus Enhancing Activities of IFITM Proteins on Entry of Human Coronaviruses, J. Virol, doi:10.1128/JVI.01535-17
Zhou, Frey, Yang, Viral calciomics: Interplays between Ca 2+ and virus, Cell Calcium, doi:10.1016/j.ceca.2009.05.005
Zhu, Yu, Hu, Wu, Chong et al., SARS-CoV-2-derived fusion inhibitor lipopeptides exhibit highly potent and broad-spectrum activity against divergent human coronaviruses, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00698-x
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3390/jcm12186079",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm12186079",
"abstract": "<jats:p>SARS-CoV-2 infection induces non-physiological syncytia when its spike fusogenic protein on the surface of the host cells interacts with the ACE2 receptor on adjacent cells. Spike-induced syncytia are beneficial for virus replication, transmission, and immune evasion, and contribute to the progression of COVID-19. In this review, we highlight the properties of viral fusion proteins, mainly the SARS-CoV-2 spike, and the involvement of the host factors in the fusion process. We also highlight the possible use of anti-fusogenic factors as an antiviral for the development of therapeutics against newly emerging SARS-CoV-2 variants and how the fusogenic property of the spike could be exploited for biomedical applications.</jats:p>",
"alternative-id": [
"jcm12186079"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5056-845X",
"affiliation": [
{
"name": "Department of Pathology, University of Cambridge, Addenbrookes Hospital, Cambridge CB2 0QQ, UK"
}
],
"authenticated-orcid": false,
"family": "Ali",
"given": "Hashim",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infection, Immunity and Inflammation Research and Teaching Department, Great Ormond Street Institute of Child Health, University College London, London WC1N 1DZ, UK"
}
],
"family": "Naseem",
"given": "Asma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7767-0158",
"affiliation": [
{
"name": "Diabetes and Obesity Research Center, NYU Grossman Long Island School of Medicine, New York, NY 11501, USA"
}
],
"authenticated-orcid": false,
"family": "Siddiqui",
"given": "Zaheenul Islam",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
9,
21
]
],
"date-time": "2023-09-21T02:38:45Z",
"timestamp": 1695263925000
},
"deposited": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T06:29:50Z",
"timestamp": 1703226590000
},
"indexed": {
"date-parts": [
[
2024,
8,
19
]
],
"date-time": "2024-08-19T17:08:31Z",
"timestamp": 1724087311497
},
"is-referenced-by-count": 2,
"issue": "18",
"issued": {
"date-parts": [
[
2023,
9,
20
]
]
},
"journal-issue": {
"issue": "18",
"published-online": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
20
]
],
"date-time": "2023-09-20T00:00:00Z",
"timestamp": 1695168000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/12/18/6079/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "6079",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
9,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
20
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1083/jcb.201901017",
"article-title": "How cells fuse",
"author": "Brukman",
"doi-asserted-by": "crossref",
"first-page": "1436",
"journal-title": "J. Cell Biol.",
"key": "ref_1",
"volume": "218",
"year": "2019"
},
{
"DOI": "10.1002/glia.22924",
"article-title": "Gap junction coupling confers isopotentiality on astrocyte syncytium",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "214",
"journal-title": "Glia",
"key": "ref_2",
"volume": "64",
"year": "2016"
},
{
"DOI": "10.1126/sciadv.aba0310",
"article-title": "TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "eaba0310",
"journal-title": "Sci. Adv.",
"key": "ref_3",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/bs.pmbts.2016.12.005",
"article-title": "Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies",
"author": "Bolze",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Prog. Mol. Biol. Transl. Sci.",
"key": "ref_4",
"volume": "145",
"year": "2017"
},
{
"DOI": "10.1262/jrd.2016-108",
"article-title": "Male infertility-related molecules involved in sperm-oocyte fusion",
"author": "Mou",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Reprod. Dev.",
"key": "ref_5",
"volume": "63",
"year": "2017"
},
{
"DOI": "10.1186/s13395-017-0149-3",
"article-title": "Myoblast fusion confusion: The resolution begins",
"author": "Sampath",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Skelet. Muscle",
"key": "ref_6",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.18632/oncotarget.25742",
"article-title": "Impact of cell fusion in myeloma marrow microenvironment on tumor progression",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "30997",
"journal-title": "Oncotarget",
"key": "ref_7",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1126/science.272.5258.115",
"article-title": "Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid",
"author": "Frankel",
"doi-asserted-by": "crossref",
"first-page": "115",
"journal-title": "Science",
"key": "ref_8",
"volume": "272",
"year": "1996"
},
{
"DOI": "10.1038/modpathol.3800725",
"article-title": "The histopathology of fatal untreated human respiratory syncytial virus infection",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "Mod. Pathol.",
"key": "ref_9",
"volume": "20",
"year": "2007"
},
{
"DOI": "10.1016/j.ebiom.2020.103104",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Bussani, R., Schneider, E., Zentilin, L., Collesi, C., Ali, H., Braga, L., Volpe, M.C., Colliva, A., Zanconati, F., and Berlot, G. (2020). Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine, 61."
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Lancet Respir. Med.",
"key": "ref_11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"article-title": "Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia",
"author": "Braga",
"doi-asserted-by": "crossref",
"first-page": "88",
"journal-title": "Nature",
"key": "ref_12",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.3389/fcvm.2022.1013262",
"article-title": "SARS-CoV-2 Spike protein activates TMEM16F-mediated platelet procoagulant activity",
"author": "Cappelletto",
"doi-asserted-by": "crossref",
"first-page": "1013262",
"journal-title": "Front. Cardiovasc. Med.",
"key": "ref_13",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.7554/eLife.65962",
"article-title": "SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation",
"author": "Sanders",
"doi-asserted-by": "crossref",
"first-page": "e65962",
"journal-title": "Elife",
"key": "ref_14",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41418-021-00782-3",
"article-title": "SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "2765",
"journal-title": "Cell. Death Differ.",
"key": "ref_15",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1126/science.abb7314",
"article-title": "Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model",
"author": "Rockx",
"doi-asserted-by": "crossref",
"first-page": "1012",
"journal-title": "Science",
"key": "ref_16",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1002/path.2276",
"article-title": "Immune activation and inflammation in HIV-1 infection: Causes and consequences",
"author": "Appay",
"doi-asserted-by": "crossref",
"first-page": "231",
"journal-title": "J. Pathol.",
"key": "ref_17",
"volume": "214",
"year": "2008"
},
{
"DOI": "10.1111/imr.12823",
"article-title": "T-cell exhaustion in HIV infection",
"author": "Fenwick",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Immunol. Rev.",
"key": "ref_18",
"volume": "292",
"year": "2019"
},
{
"DOI": "10.1073/pnas.2111400119",
"article-title": "SARS-CoV-2 spreads through cell-to-cell transmission",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "e2111400119",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_19",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1128/JVI.00078-07",
"article-title": "Cell-cell fusion induced by measles virus amplifies the type I interferon response",
"author": "Herschke",
"doi-asserted-by": "crossref",
"first-page": "12859",
"journal-title": "J. Virol.",
"key": "ref_20",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1126/scisignal.abg8744",
"article-title": "SARS-CoV-2 spike protein-induced cell fusion activates the cGAS-STING pathway and the interferon response",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "eabg8744",
"journal-title": "Sci. Signal.",
"key": "ref_21",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1007/s00414-020-02500-z",
"article-title": "SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study",
"author": "Gauchotte",
"doi-asserted-by": "crossref",
"first-page": "577",
"journal-title": "Int. J. Legal Med.",
"key": "ref_22",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1016/j.hrthm.2020.05.001",
"article-title": "Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management",
"author": "Siripanthong",
"doi-asserted-by": "crossref",
"first-page": "1463",
"journal-title": "Heart Rhythm.",
"key": "ref_23",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.7150/ijbs.69677",
"article-title": "Manifestations and Mechanism of SARS-CoV2 Mediated Cardiac Injury",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "2703",
"journal-title": "Int. J. Biol. Sci.",
"key": "ref_24",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1128/JVI.01368-21",
"article-title": "Highly Efficient SARS-CoV-2 Infection of Human Cardiomyocytes: Spike Protein-Mediated Cell Fusion and Its Inhibition",
"author": "Navaratnarajah",
"doi-asserted-by": "crossref",
"first-page": "e0136821",
"journal-title": "J. Virol.",
"key": "ref_25",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0282151",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Clemens, D.J., Ye, D., Zhou, W., Kim, C.S.J., Pease, D.R., Navaratnarajah, C.K., Barkhymer, A., Tester, D.J., Nelson, T.J., and Cattaneo, R. (2023). SARS-CoV-2 spike protein-mediated cardiomyocyte fusion may contribute to increased arrhythmic risk in COVID-19. PLoS ONE, 18."
},
{
"DOI": "10.1111/febs.15146",
"article-title": "Cardiac regeneration and remodelling of the cardiomyocyte cytoarchitecture",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "FEBS J.",
"key": "ref_27",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa030747",
"article-title": "Identification of a novel coronavirus in patients with severe acute respiratory syndrome",
"author": "Drosten",
"doi-asserted-by": "crossref",
"first-page": "1967",
"journal-title": "N. Engl. J. Med.",
"key": "ref_28",
"volume": "348",
"year": "2003"
},
{
"DOI": "10.3201/eid2607.200092",
"article-title": "Possible Bat Origin of Severe Acute Respiratory Syndrome Coronavirus 2",
"author": "Lau",
"doi-asserted-by": "crossref",
"first-page": "1542",
"journal-title": "Emerg. Infect. Dis.",
"key": "ref_29",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1211721",
"article-title": "Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia",
"author": "Zaki",
"doi-asserted-by": "crossref",
"first-page": "1814",
"journal-title": "N. Engl. J. Med.",
"key": "ref_30",
"volume": "367",
"year": "2012"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_31",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/nature02145",
"article-title": "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "450",
"journal-title": "Nature",
"key": "ref_32",
"volume": "426",
"year": "2003"
},
{
"DOI": "10.1038/nature12328",
"article-title": "Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "227",
"journal-title": "Nature",
"key": "ref_33",
"volume": "500",
"year": "2013"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"article-title": "Coronavirus biology and replication: Implications for SARS-CoV-2",
"author": "Kratzel",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_34",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"article-title": "A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Mol. Cell.",
"key": "ref_35",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.3390/v12070693",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Barrett, C.T., and Dutch, R.E. (2020). Viral Membrane Fusion and the Transmembrane Domain. Viruses, 12."
},
{
"DOI": "10.1038/90341",
"article-title": "Implications of a fusion peptide structure",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "653",
"journal-title": "Nat. Struct. Biol.",
"key": "ref_37",
"volume": "8",
"year": "2001"
},
{
"DOI": "10.1146/annurev-virology-092818-015523",
"article-title": "Fusogenic Reoviruses and Their Fusion-Associated Small Transmembrane (FAST) Proteins",
"author": "Duncan",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "Annu. Rev. Virol.",
"key": "ref_38",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.3389/fchem.2021.689006",
"article-title": "Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses",
"author": "Lozada",
"doi-asserted-by": "crossref",
"first-page": "689006",
"journal-title": "Front. Chem.",
"key": "ref_39",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/ijms21249644",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Leroy, H., Han, M., Woottum, M., Bracq, L., Bouchet, J., Xie, M., and Benichou, S. (2020). Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.1038/nrmicro1326",
"article-title": "Virus membrane-fusion proteins: More than one way to make a hairpin",
"author": "Kielian",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_41",
"volume": "4",
"year": "2006"
},
{
"DOI": "10.1016/j.virol.2015.03.043",
"article-title": "Viral membrane fusion",
"author": "Harrison",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "Virology",
"key": "ref_42",
"volume": "479–480",
"year": "2015"
},
{
"DOI": "10.1146/annurev-cellbio-101512-122422",
"article-title": "Virus and cell fusion mechanisms",
"author": "Podbilewicz",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Annu. Rev. Cell. Dev. Biol.",
"key": "ref_43",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1016/j.sbi.2009.02.012",
"article-title": "Class III viral membrane fusion proteins",
"author": "Backovic",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Curr. Opin. Struct. Biol.",
"key": "ref_44",
"volume": "19",
"year": "2009"
},
{
"DOI": "10.1038/371037a0",
"article-title": "Structure of influenza haemagglutinin at the pH of membrane fusion",
"author": "Bullough",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Nature",
"key": "ref_45",
"volume": "371",
"year": "1994"
},
{
"DOI": "10.1073/pnas.96.16.8967",
"article-title": "N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "8967",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_46",
"volume": "96",
"year": "1999"
},
{
"DOI": "10.1371/journal.ppat.1007675",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Kanai, Y., Kawagishi, T., Sakai, Y., Nouda, R., Shimojima, M., Saijo, M., Matsuura, Y., and Kobayashi, T. (2019). Cell-cell fusion induced by reovirus FAST proteins enhances replication and pathogenicity of non-enveloped dsRNA viruses. PLoS Pathog., 15."
},
{
"DOI": "10.1016/j.tim.2014.08.005",
"article-title": "Reovirus FAST proteins: Virus-encoded cellular fusogens",
"author": "Ciechonska",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Trends Microbiol.",
"key": "ref_48",
"volume": "22",
"year": "2014"
},
{
"DOI": "10.1371/journal.ppat.1000016",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Salsman, J., Top, D., Barry, C., and Duncan, R. (2008). A virus-encoded cell-cell fusion machine dependent on surrogate adhesins. PLoS Pathog., 4."
},
{
"DOI": "10.1128/JVI.78.6.2808-2818.2004",
"article-title": "Structural and functional properties of an unusual internal fusion peptide in a nonenveloped virus membrane fusion protein",
"author": "Shmulevitz",
"doi-asserted-by": "crossref",
"first-page": "2808",
"journal-title": "J. Virol.",
"key": "ref_50",
"volume": "78",
"year": "2004"
},
{
"DOI": "10.7554/eLife.51358",
"article-title": "A viral fusogen hijacks the actin cytoskeleton to drive cell-cell fusion",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "e51358",
"journal-title": "Elife",
"key": "ref_51",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1021/bi00608a034",
"article-title": "Measurement of repulsive forces between charged phospholipid bilayers",
"author": "Cowley",
"doi-asserted-by": "crossref",
"first-page": "3163",
"journal-title": "Biochemistry",
"key": "ref_52",
"volume": "17",
"year": "1978"
},
{
"DOI": "10.1126/science.2814514",
"article-title": "Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers",
"author": "Helm",
"doi-asserted-by": "crossref",
"first-page": "919",
"journal-title": "Science",
"key": "ref_53",
"volume": "246",
"year": "1989"
},
{
"DOI": "10.1073/pnas.2007526118",
"article-title": "Evolutionarily related small viral fusogens hijack distinct but modular actin nucleation pathways to drive cell-cell fusion",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "e2007526118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_54",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1007/s00018-004-4006-2",
"article-title": "Structure-based models of cadherin-mediated cell adhesion: The evolution continues",
"author": "Koch",
"doi-asserted-by": "crossref",
"first-page": "1884",
"journal-title": "Cell. Mol. Life Sci.",
"key": "ref_55",
"volume": "61",
"year": "2004"
},
{
"DOI": "10.1128/JVI.03372-12",
"article-title": "TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium",
"author": "Bertram",
"doi-asserted-by": "crossref",
"first-page": "6150",
"journal-title": "J. Virol.",
"key": "ref_56",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1146/annurev.biochem.70.1.777",
"article-title": "Mechanisms of viral membrane fusion and its inhibition",
"author": "Eckert",
"doi-asserted-by": "crossref",
"first-page": "777",
"journal-title": "Annu. Rev. Biochem.",
"key": "ref_57",
"volume": "70",
"year": "2001"
},
{
"DOI": "10.1038/nsmb.1456",
"article-title": "Viral membrane fusion",
"author": "Harrison",
"doi-asserted-by": "crossref",
"first-page": "690",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_58",
"volume": "15",
"year": "2008"
},
{
"DOI": "10.1038/s41467-021-25589-1",
"article-title": "Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation",
"author": "Welch",
"doi-asserted-by": "crossref",
"first-page": "5333",
"journal-title": "Nat. Commun.",
"key": "ref_59",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"article-title": "Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Nature",
"key": "ref_60",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1002/mco2.79",
"article-title": "Potent neutralizing RBD-specific antibody cocktail against SARS-CoV-2 and its mutant",
"author": "Jia",
"doi-asserted-by": "crossref",
"first-page": "442",
"journal-title": "MedComm",
"key": "ref_61",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03807-6",
"article-title": "SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape",
"author": "Starr",
"doi-asserted-by": "crossref",
"first-page": "97",
"journal-title": "Nature",
"key": "ref_62",
"volume": "597",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2772-0",
"article-title": "Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion",
"author": "Benton",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Nature",
"key": "ref_63",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00079-09",
"article-title": "Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide",
"author": "Madu",
"doi-asserted-by": "crossref",
"first-page": "7411",
"journal-title": "J. Virol.",
"key": "ref_64",
"volume": "83",
"year": "2009"
},
{
"DOI": "10.1038/s41586-020-2179-y",
"article-title": "Structural basis of receptor recognition by SARS-CoV-2",
"author": "Shang",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Nature",
"key": "ref_65",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell. Biol.",
"key": "ref_66",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1038/ncomms15672",
"article-title": "A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "15672",
"journal-title": "Nat. Commun.",
"key": "ref_67",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(04)15788-7",
"article-title": "Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "938",
"journal-title": "Lancet",
"key": "ref_68",
"volume": "363",
"year": "2004"
},
{
"DOI": "10.1016/j.cell.2020.07.012",
"article-title": "The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1284",
"journal-title": "Cell",
"key": "ref_69",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1016/j.molcel.2020.07.027",
"article-title": "Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting",
"author": "Robson",
"doi-asserted-by": "crossref",
"first-page": "710",
"journal-title": "Mol. Cell.",
"key": "ref_70",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"article-title": "Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus",
"author": "Korber",
"doi-asserted-by": "crossref",
"first-page": "812",
"journal-title": "Cell",
"key": "ref_71",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1128/mBio.00587-21",
"doi-asserted-by": "crossref",
"key": "ref_72",
"unstructured": "Cheng, Y.W., Chao, T.L., Li, C.L., Wang, S.H., Kao, H.C., Tsai, Y.M., Wang, H.Y., Hsieh, C.L., Lin, Y.Y., and Chen, P.J. (2021). D614G Substitution of SARS-CoV-2 Spike Protein Increases Syncytium Formation and Virus Titer via Enhanced Furin-Mediated Spike Cleavage. mBio, 12."
},
{
"DOI": "10.1038/s41586-021-03944-y",
"article-title": "SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion",
"author": "Mlcochova",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Nature",
"key": "ref_73",
"volume": "599",
"year": "2021"
},
{
"DOI": "10.15252/embj.2021108944",
"article-title": "SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation",
"author": "Rajah",
"doi-asserted-by": "crossref",
"first-page": "e108944",
"journal-title": "EMBO J.",
"key": "ref_74",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04462-1",
"article-title": "Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant",
"author": "Suzuki",
"doi-asserted-by": "crossref",
"first-page": "700",
"journal-title": "Nature",
"key": "ref_75",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01397-4",
"article-title": "SARS-CoV-2 variants of concern are emerging in India",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "1131",
"journal-title": "Nat. Med.",
"key": "ref_76",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04266-9",
"article-title": "Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation",
"author": "Saito",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Nature",
"key": "ref_77",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"article-title": "Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Nature",
"key": "ref_78",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01290-3",
"article-title": "Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination",
"author": "Wall",
"doi-asserted-by": "crossref",
"first-page": "2331",
"journal-title": "Lancet",
"key": "ref_79",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.05.022",
"article-title": "Novel SARS-CoV-2 variants: The pandemics within the pandemic",
"author": "Boehm",
"doi-asserted-by": "crossref",
"first-page": "1109",
"journal-title": "Clin. Microbiol. Infect.",
"key": "ref_80",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1074/jbc.M111.287912",
"article-title": "A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing",
"author": "Schjoldager",
"doi-asserted-by": "crossref",
"first-page": "40122",
"journal-title": "J. Biol. Chem.",
"key": "ref_81",
"volume": "286",
"year": "2011"
},
{
"DOI": "10.1073/pnas.2109905118",
"article-title": "Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "e2109905118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_82",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-00121-z",
"article-title": "Fast-spreading COVID variant can elude immune responses",
"author": "Callaway",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Nature",
"key": "ref_83",
"volume": "589",
"year": "2021"
},
{
"DOI": "10.1126/science.abn7760",
"article-title": "SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein-ACE2 complex",
"author": "Mannar",
"doi-asserted-by": "crossref",
"first-page": "760",
"journal-title": "Science",
"key": "ref_84",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1128/JVI.02146-06",
"article-title": "The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein",
"author": "McBride",
"doi-asserted-by": "crossref",
"first-page": "2418",
"journal-title": "J. Virol.",
"key": "ref_85",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1074/jbc.RA120.016175",
"doi-asserted-by": "crossref",
"key": "ref_86",
"unstructured": "Boson, B., Legros, V., Zhou, B., Siret, E., Mathieu, C., Cosset, F.L., Lavillette, D., and Denolly, S. (2021). The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem., 296."
},
{
"DOI": "10.1128/mBio.00788-21",
"doi-asserted-by": "crossref",
"key": "ref_87",
"unstructured": "Rocheleau, L., Laroche, G., Fu, K., Stewart, C.M., Mohamud, A.O., Cote, M., Giguere, P.M., Langlois, M.A., and Pelchat, M. (2021). Identification of a High-Frequency Intrahost SARS-CoV-2 Spike Variant with Enhanced Cytopathic and Fusogenic Effects. mBio, 12."
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "ref_88",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/nm1267",
"article-title": "A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury",
"author": "Kuba",
"doi-asserted-by": "crossref",
"first-page": "875",
"journal-title": "Nat. Med.",
"key": "ref_89",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.1126/science.abb2762",
"article-title": "Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "1444",
"journal-title": "Science",
"key": "ref_90",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1038/s41564-021-00908-w",
"article-title": "The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets",
"author": "Peacock",
"doi-asserted-by": "crossref",
"first-page": "899",
"journal-title": "Nat. Microbiol.",
"key": "ref_91",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1007/s10096-008-0495-5",
"article-title": "siRNA silencing of angiotensin-converting enzyme 2 reduced severe acute respiratory syndrome-associated coronavirus replications in Vero E6 cells",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "709",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "ref_92",
"volume": "27",
"year": "2008"
},
{
"DOI": "10.1038/nrm934",
"article-title": "Furin at the cutting edge: From protein traffic to embryogenesis and disease",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "753",
"journal-title": "Nat. Rev. Mol. Cell. Biol.",
"key": "ref_93",
"volume": "3",
"year": "2002"
},
{
"DOI": "10.1002/cti2.1073",
"article-title": "Furin-mediated protein processing in infectious diseases and cancer",
"author": "Braun",
"doi-asserted-by": "crossref",
"first-page": "e1073",
"journal-title": "Clin. Transl. Immunol.",
"key": "ref_94",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.3390/v11090837",
"doi-asserted-by": "crossref",
"key": "ref_95",
"unstructured": "Izaguirre, G. (2019). The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses, 11."
},
{
"DOI": "10.1128/JVI.00613-09",
"article-title": "Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells",
"author": "Yamada",
"doi-asserted-by": "crossref",
"first-page": "8744",
"journal-title": "J. Virol.",
"key": "ref_96",
"volume": "83",
"year": "2009"
},
{
"DOI": "10.1016/j.antiviral.2020.104742",
"article-title": "The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade",
"author": "Coutard",
"doi-asserted-by": "crossref",
"first-page": "104742",
"journal-title": "Antiviral Res.",
"key": "ref_97",
"volume": "176",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03237-4",
"article-title": "Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "Nature",
"key": "ref_98",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2022.2114850",
"article-title": "Furin cleavage is required for swine acute diarrhea syndrome coronavirus spike protein-mediated cell-cell fusion",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "2176",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_99",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2020.108254",
"article-title": "Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "108254",
"journal-title": "Cell. Rep.",
"key": "ref_100",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1004048",
"doi-asserted-by": "crossref",
"key": "ref_101",
"unstructured": "Desai, T.M., Marin, M., Chin, C.R., Savidis, G., Brass, A.L., and Melikyan, G.B. (2014). IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog., 10."
},
{
"DOI": "10.1371/journal.ppat.1001258",
"doi-asserted-by": "crossref",
"key": "ref_102",
"unstructured": "Huang, I.C., Bailey, C.C., Weyer, J.L., Radoshitzky, S.R., Becker, M.M., Chiang, J.J., Brass, A.L., Ahmed, A.A., Chi, X., and Dong, L. (2011). Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog., 7."
},
{
"DOI": "10.1016/0092-8674(84)90270-8",
"article-title": "Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells",
"author": "Friedman",
"doi-asserted-by": "crossref",
"first-page": "745",
"journal-title": "Cell",
"key": "ref_103",
"volume": "38",
"year": "1984"
},
{
"DOI": "10.1186/1471-2164-13-155",
"doi-asserted-by": "crossref",
"key": "ref_104",
"unstructured": "Hickford, D., Frankenberg, S., Shaw, G., and Renfree, M.B. (2012). Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genom., 13."
},
{
"DOI": "10.1128/JVI.03382-12",
"article-title": "IFITM-2 and IFITM-3 but not IFITM-1 restrict Rift Valley fever virus",
"author": "Mudhasani",
"doi-asserted-by": "crossref",
"first-page": "8451",
"journal-title": "J. Virol.",
"key": "ref_105",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1371/journal.pone.0104341",
"doi-asserted-by": "crossref",
"key": "ref_106",
"unstructured": "Weston, S., Czieso, S., White, I.J., Smith, S.E., Kellam, P., and Marsh, M. (2014). A membrane topology model for human interferon inducible transmembrane protein 1. PLoS ONE, 9."
},
{
"DOI": "10.3390/v6093683",
"article-title": "IFITM proteins inhibit entry driven by the MERS-coronavirus spike protein: Evidence for cholesterol-independent mechanisms",
"author": "Wrensch",
"doi-asserted-by": "crossref",
"first-page": "3683",
"journal-title": "Viruses",
"key": "ref_107",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1073/pnas.1320856111",
"article-title": "Interferon induction of IFITM proteins promotes infection by human coronavirus OC43",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "6756",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_108",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1128/JVI.01535-17",
"article-title": "Identification of Residues Controlling Restriction versus Enhancing Activities of IFITM Proteins on Entry of Human Coronaviruses",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "e01535-17",
"journal-title": "J. Virol.",
"key": "ref_109",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.15252/embj.2020106501",
"article-title": "Opposing activities of IFITM proteins in SARS-CoV-2 infection",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "e106501",
"journal-title": "EMBO J.",
"key": "ref_110",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.chom.2013.03.006",
"article-title": "The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "452",
"journal-title": "Cell Host Microbe",
"key": "ref_111",
"volume": "13",
"year": "2013"
},
{
"DOI": "10.1146/annurev-virology-031413-085537",
"article-title": "IFITM-Family Proteins: The Cell’s First Line of Antiviral Defense",
"author": "Bailey",
"doi-asserted-by": "crossref",
"first-page": "261",
"journal-title": "Annu. Rev. Virol.",
"key": "ref_112",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.1007/s40588-018-0103-0",
"article-title": "Antiviral Protection by IFITM3 In Vivo",
"author": "Zani",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "Curr. Clin. Microbiol. Rep.",
"key": "ref_113",
"volume": "5",
"year": "2018"
},
{
"DOI": "10.15252/embj.2020106267",
"article-title": "Syncytia formation by SARS-CoV-2-infected cells",
"author": "Buchrieser",
"doi-asserted-by": "crossref",
"first-page": "e106267",
"journal-title": "EMBO J.",
"key": "ref_114",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1084/jem.20161270",
"article-title": "ZMPSTE24 defends against influenza and other pathogenic viruses",
"author": "Fu",
"doi-asserted-by": "crossref",
"first-page": "919",
"journal-title": "J. Exp. Med.",
"key": "ref_115",
"volume": "214",
"year": "2017"
},
{
"DOI": "10.1089/dna.2017.3791",
"article-title": "ZMPSTE24 Is Downstream Effector of Interferon-Induced Transmembrane Antiviral Activity",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "513",
"journal-title": "DNA Cell. Biol.",
"key": "ref_116",
"volume": "36",
"year": "2017"
},
{
"DOI": "10.1128/mbio.02543-22",
"doi-asserted-by": "crossref",
"key": "ref_117",
"unstructured": "Shilagardi, K., Spear, E.D., Abraham, R., Griffin, D.E., and Michaelis, S. (2022). The Integral Membrane Protein ZMPSTE24 Protects Cells from SARS-CoV-2 Spike-Mediated Pseudovirus Infection and Syncytia Formation. mBio, 13."
},
{
"DOI": "10.3390/v12060629",
"doi-asserted-by": "crossref",
"key": "ref_118",
"unstructured": "Yamamoto, M., Kiso, M., Sakai-Tagawa, Y., Iwatsuki-Horimoto, K., Imai, M., Takeda, M., Kinoshita, N., Ohmagari, N., Gohda, J., and Semba, K. (2020). The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses, 12."
},
{
"DOI": "10.1128/AAC.01043-16",
"article-title": "Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "6532",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_119",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.1091/mbc.e08-11-1135",
"article-title": "ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha",
"author": "Bobe",
"doi-asserted-by": "crossref",
"first-page": "1785",
"journal-title": "Mol. Biol. Cell.",
"key": "ref_120",
"volume": "20",
"year": "2009"
},
{
"DOI": "10.1128/JVI.02202-13",
"article-title": "TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein",
"author": "Heurich",
"doi-asserted-by": "crossref",
"first-page": "1293",
"journal-title": "J. Virol.",
"key": "ref_121",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1074/jbc.M505111200",
"article-title": "Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2)",
"author": "Lambert",
"doi-asserted-by": "crossref",
"first-page": "30113",
"journal-title": "J. Biol. Chem.",
"key": "ref_122",
"volume": "280",
"year": "2005"
},
{
"DOI": "10.1038/ncomms5058",
"article-title": "ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A",
"author": "Romi",
"doi-asserted-by": "crossref",
"first-page": "4058",
"journal-title": "Nat. Commun.",
"key": "ref_123",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0073296",
"doi-asserted-by": "crossref",
"key": "ref_124",
"unstructured": "Houri, N., Huang, K.C., and Nalbantoglu, J. (2013). The Coxsackievirus and Adenovirus Receptor (CAR) undergoes ectodomain shedding and regulated intramembrane proteolysis (RIP). PLoS ONE, 8."
},
{
"DOI": "10.1038/nm.1961",
"article-title": "Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71",
"author": "Nishimura",
"doi-asserted-by": "crossref",
"first-page": "794",
"journal-title": "Nat. Med.",
"key": "ref_125",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.15252/embr.202154305",
"article-title": "ADAM10 and ADAM17 promote SARS-CoV-2 cell entry and spike protein-mediated lung cell fusion",
"author": "Jocher",
"doi-asserted-by": "crossref",
"first-page": "e54305",
"journal-title": "EMBO Rep.",
"key": "ref_126",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"article-title": "An inflammatory cytokine signature predicts COVID-19 severity and survival",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "1636",
"journal-title": "Nat. Med.",
"key": "ref_127",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes",
"author": "Karki",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Cell",
"key": "ref_128",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/ncprheum0797",
"article-title": "Drug insight: Tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis",
"author": "Moss",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Nat. Clin. Pract. Rheumatol.",
"key": "ref_129",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.2174/138161209788682398",
"article-title": "ADAM17 as a therapeutic target in multiple diseases",
"author": "Arribas",
"doi-asserted-by": "crossref",
"first-page": "2319",
"journal-title": "Curr. Pharm. Des.",
"key": "ref_130",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1038/s41467-020-18168-3",
"article-title": "Dynamic remodelling of the human host cell proteome and phosphoproteome upon enterovirus infection",
"author": "Giansanti",
"doi-asserted-by": "crossref",
"first-page": "4332",
"journal-title": "Nat. Commun.",
"key": "ref_131",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/v12070727",
"doi-asserted-by": "crossref",
"key": "ref_132",
"unstructured": "Zhao, J., Chen, J., Li, M., Chen, M., and Sun, C. (2020). Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities. Viruses, 12."
},
{
"DOI": "10.1038/s41564-020-0701-5",
"article-title": "Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol",
"author": "Abrams",
"doi-asserted-by": "crossref",
"first-page": "929",
"journal-title": "Nat. Microbiol.",
"key": "ref_133",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1114981109",
"article-title": "Systematic identification of type I and type II interferon-induced antiviral factors",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "4239",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_134",
"volume": "109",
"year": "2012"
},
{
"DOI": "10.15252/embj.2020106057",
"article-title": "Cholesterol 25-Hydroxylase inhibits SARS-CoV-2 and other coronaviruses by depleting membrane cholesterol",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "e106057",
"journal-title": "EMBO J.",
"key": "ref_135",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2012197117",
"article-title": "Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion",
"author": "Zang",
"doi-asserted-by": "crossref",
"first-page": "32105",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_136",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0769-y",
"article-title": "LY6E impairs coronavirus fusion and confers immune control of viral disease",
"author": "Pfaender",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "Nat. Microbiol.",
"key": "ref_137",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/nature09907",
"article-title": "A diverse range of gene products are effectors of the type I interferon antiviral response",
"author": "Schoggins",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Nature",
"key": "ref_138",
"volume": "472",
"year": "2011"
},
{
"DOI": "10.1016/j.cell.2012.07.036",
"article-title": "TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Cell",
"key": "ref_139",
"volume": "151",
"year": "2012"
},
{
"DOI": "10.1021/acsinfecdis.0c00052",
"article-title": "Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "909",
"journal-title": "ACS Infect. Dis.",
"key": "ref_140",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1717956115",
"article-title": "Single-molecule analysis of phospholipid scrambling by TMEM16F",
"author": "Watanabe",
"doi-asserted-by": "crossref",
"first-page": "3066",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_141",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1128/JVI.03287-13",
"article-title": "Role of phosphatidylserine receptors in enveloped virus infection",
"author": "Morizono",
"doi-asserted-by": "crossref",
"first-page": "4275",
"journal-title": "J. Virol.",
"key": "ref_142",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1016/j.jbc.2021.100411",
"doi-asserted-by": "crossref",
"key": "ref_143",
"unstructured": "Whitlock, J.M., and Chernomordik, L.V. (2021). Flagging fusion: Phosphatidylserine signaling in cell-cell fusion. J. Biol. Chem., 296."
},
{
"DOI": "10.4049/jimmunol.170.9.4840",
"article-title": "Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells",
"author": "Callahan",
"doi-asserted-by": "crossref",
"first-page": "4840",
"journal-title": "J. Immunol.",
"key": "ref_144",
"volume": "170",
"year": "2003"
},
{
"DOI": "10.1016/j.chom.2017.06.012",
"article-title": "Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine",
"author": "Zaitseva",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Cell Host Microbe",
"key": "ref_145",
"volume": "22",
"year": "2017"
},
{
"DOI": "10.1371/journal.ppat.1006848",
"doi-asserted-by": "crossref",
"key": "ref_146",
"unstructured": "Nanbo, A., Maruyama, J., Imai, M., Ujie, M., Fujioka, Y., Nishide, S., Takada, A., Ohba, Y., and Kawaoka, Y. (2018). Ebola virus requires a host scramblase for externalization of phosphatidylserine on the surface of viral particles. PLoS Pathog., 14."
},
{
"DOI": "10.1128/mBio.01552-15",
"article-title": "Binding of alphaherpesvirus glycoprotein H to surface alpha4beta1-integrins activates calcium-signaling pathways and induces phosphatidylserine exposure on the plasma membrane",
"author": "Azab",
"doi-asserted-by": "crossref",
"first-page": "e01552-15",
"journal-title": "mBio",
"key": "ref_147",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1159/000133969",
"article-title": "The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25",
"author": "Calabrese",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Cytogenet. Cell Genet.",
"key": "ref_148",
"volume": "69",
"year": "1995"
},
{
"DOI": "10.1093/emboj/17.6.1606",
"article-title": "Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin",
"author": "Sasaki",
"doi-asserted-by": "crossref",
"first-page": "1606",
"journal-title": "EMBO J.",
"key": "ref_149",
"volume": "17",
"year": "1998"
},
{
"DOI": "10.1002/cam4.2075",
"article-title": "Increased LGALS3 expression independently predicts shorter overall survival in patients with the proneural subtype of glioblastoma",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "2031",
"journal-title": "Cancer Med.",
"key": "ref_150",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1002/art.38065",
"article-title": "Unique protein signature of circulating microparticles in systemic lupus erythematosus",
"author": "Ostergaard",
"doi-asserted-by": "crossref",
"first-page": "2680",
"journal-title": "Arthritis Rheum.",
"key": "ref_151",
"volume": "65",
"year": "2013"
},
{
"article-title": "90k is a serum marker of poor-prognosis in non-hodgkins-lymphoma patients",
"author": "Rea",
"first-page": "723",
"journal-title": "Oncol. Rep.",
"key": "ref_152",
"volume": "1",
"year": "1994"
},
{
"DOI": "10.1016/S0168-8278(96)80076-6",
"article-title": "Elevated serum levels of 90K/MAC-2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients",
"author": "Artini",
"doi-asserted-by": "crossref",
"first-page": "212",
"journal-title": "J. Hepatol.",
"key": "ref_153",
"volume": "25",
"year": "1996"
},
{
"DOI": "10.1099/vir.0.066837-0",
"article-title": "Acute hantavirus infection induces galectin-3-binding protein",
"author": "Hepojoki",
"doi-asserted-by": "crossref",
"first-page": "2356",
"journal-title": "J. Gen. Virol.",
"key": "ref_154",
"volume": "95",
"year": "2014"
},
{
"DOI": "10.3390/ijms17060832",
"doi-asserted-by": "crossref",
"key": "ref_155",
"unstructured": "Liu, K.T., Liu, Y.H., Chen, Y.H., Lin, C.Y., Huang, C.H., Yen, M.C., and Kuo, P.L. (2016). Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection. Int. J. Mol. Sci., 17."
},
{
"DOI": "10.1093/infdis/164.3.616",
"article-title": "Unusually high level of a tumor-associated antigen in the serum of human immunodeficiency virus-seropositive individuals",
"author": "Natoli",
"doi-asserted-by": "crossref",
"first-page": "616",
"journal-title": "J. Infect. Dis.",
"key": "ref_156",
"volume": "164",
"year": "1991"
},
{
"DOI": "10.1002/art.24574",
"article-title": "Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways",
"author": "Filer",
"doi-asserted-by": "crossref",
"first-page": "1604",
"journal-title": "Arthritis Rheum.",
"key": "ref_157",
"volume": "60",
"year": "2009"
},
{
"article-title": "Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion",
"author": "Inohara",
"first-page": "4530",
"journal-title": "Cancer Res.",
"key": "ref_158",
"volume": "56",
"year": "1996"
},
{
"article-title": "Increased Gal-3BP plasma levels in hospitalized patients infected with SARS-CoV-2",
"author": "Gallo",
"first-page": "151",
"journal-title": "Clin. Exp. Med.",
"key": "ref_159",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.15252/emmm.202114167",
"article-title": "High-resolution serum proteome trajectories in COVID-19 reveal patient-specific seroconversion",
"author": "Geyer",
"doi-asserted-by": "crossref",
"first-page": "e14167",
"journal-title": "EMBO Mol. Med.",
"key": "ref_160",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/biom11081136",
"doi-asserted-by": "crossref",
"key": "ref_161",
"unstructured": "Kusnierz-Cabala, B., Maziarz, B., Dumnicka, P., Dembinski, M., Kapusta, M., Bociaga-Jasik, M., Winiarski, M., Garlicki, A., Grodzicki, T., and Kukla, M. (2021). Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study. Biomolecules, 11."
},
{
"DOI": "10.1016/j.cels.2020.05.012",
"article-title": "Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection",
"author": "Messner",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Cell. Syst.",
"key": "ref_162",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-23494-1",
"article-title": "SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care",
"author": "Gutmann",
"doi-asserted-by": "crossref",
"first-page": "3406",
"journal-title": "Nat. Commun.",
"key": "ref_163",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.1009127",
"doi-asserted-by": "crossref",
"key": "ref_164",
"unstructured": "Dias, S.S.G., Soares, V.C., Ferreira, A.C., Sacramento, C.Q., Fintelman-Rodrigues, N., Temerozo, J.R., Teixeira, L., Nunes da Silva, M.A., Barreto, E., and Mattos, M. (2020). Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog., 16."
},
{
"DOI": "10.1101/cshperspect.a004820",
"doi-asserted-by": "crossref",
"key": "ref_165",
"unstructured": "Lorizate, M., and Krausslich, H.G. (2011). Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol., 3."
},
{
"DOI": "10.1016/j.jlr.2021.100129",
"article-title": "The roles of lipids in SARS-CoV-2 viral replication and the host immune response",
"author": "Theken",
"doi-asserted-by": "crossref",
"first-page": "100129",
"journal-title": "J. Lipid Res.",
"key": "ref_166",
"volume": "62",
"year": "2021"
},
{
"DOI": "10.1016/j.jbc.2021.100470",
"doi-asserted-by": "crossref",
"key": "ref_167",
"unstructured": "Vitner, E.B., Achdout, H., Avraham, R., Politi, B., Cherry, L., Tamir, H., Yahalom-Ronen, Y., Paran, N., Melamed, S., and Erez, N. (2021). Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J. Biol. Chem., 296."
},
{
"DOI": "10.1093/braincomms/fcad086",
"article-title": "Antiviral activity of glucosylceramide synthase inhibitors in alphavirus infection of the central nervous system",
"author": "Avraham",
"doi-asserted-by": "crossref",
"first-page": "fcad086",
"journal-title": "Brain Commun.",
"key": "ref_168",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1074/jbc.M114.574285",
"article-title": "Sphingomyelin synthase 2, but not sphingomyelin synthase 1, is involved in HIV-1 envelope-mediated membrane fusion",
"author": "Hayashi",
"doi-asserted-by": "crossref",
"first-page": "30842",
"journal-title": "J. Biol. Chem.",
"key": "ref_169",
"volume": "289",
"year": "2014"
},
{
"DOI": "10.1016/j.chemphyslip.2015.07.025",
"article-title": "Inhibitors of dihydroceramide desaturase 1: Therapeutic agents and pharmacological tools to decipher the role of dihydroceramides in cell biology",
"author": "Casasampere",
"doi-asserted-by": "crossref",
"first-page": "33",
"journal-title": "Chem. Phys. Lipids",
"key": "ref_170",
"volume": "197",
"year": "2016"
},
{
"DOI": "10.1128/JVI.00807-21",
"article-title": "N-(4-Hydroxyphenyl) Retinamide Suppresses SARS-CoV-2 Spike Protein-Mediated Cell-Cell Fusion by a Dihydroceramide Delta4-Desaturase 1-Independent Mechanism",
"author": "Hayashi",
"doi-asserted-by": "crossref",
"first-page": "e0080721",
"journal-title": "J. Virol.",
"key": "ref_171",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1038/sj.embor.7400921",
"article-title": "Calcium: A fundamental regulator of intracellular membrane fusion?",
"author": "Hay",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "EMBO Rep.",
"key": "ref_172",
"volume": "8",
"year": "2007"
},
{
"DOI": "10.1007/978-3-030-12457-1_1",
"article-title": "Calcium Signaling: From Basic to Bedside",
"author": "Islam",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "ref_173",
"volume": "1131",
"year": "2020"
},
{
"DOI": "10.3390/cells9010094",
"doi-asserted-by": "crossref",
"key": "ref_174",
"unstructured": "Chen, X., Cao, R., and Zhong, W. (2019). Host Calcium Channels and Pumps in Viral Infections. Cells, 9."
},
{
"DOI": "10.1016/j.mam.2021.101004",
"article-title": "Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance",
"author": "Saurav",
"doi-asserted-by": "crossref",
"first-page": "101004",
"journal-title": "Mol. Aspects Med.",
"key": "ref_175",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1016/j.ceca.2009.05.005",
"article-title": "Viral calciomics: Interplays between Ca2+ and virus",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Cell Calcium",
"key": "ref_176",
"volume": "46",
"year": "2009"
},
{
"DOI": "10.3389/fcimb.2022.845580",
"doi-asserted-by": "crossref",
"key": "ref_177",
"unstructured": "Osorio, C., Sfera, A., Anton, J.J., Thomas, K.G., Andronescu, C.V., Li, E., Yahia, R.W., Avalos, A.G., and Kozlakidis, Z. (2022). Virus-Induced Membrane Fusion in Neurodegenerative Disorders. Front. Cell. Infect. Microbiol., 12."
},
{
"DOI": "10.1371/journal.pbio.3000626",
"doi-asserted-by": "crossref",
"key": "ref_178",
"unstructured": "Das, D.K., Bulow, U., Diehl, W.E., Durham, N.D., Senjobe, F., Chandran, K., Luban, J., and Munro, J.B. (2020). Conformational changes in the Ebola virus membrane fusion machine induced by pH, Ca2+, and receptor binding. PLoS Biol., 18."
},
{
"DOI": "10.1371/journal.ppat.1004530",
"doi-asserted-by": "crossref",
"key": "ref_179",
"unstructured": "Dube, M., Rey, F.A., and Kielian, M. (2014). Rubella virus: First calcium-requiring viral fusion protein. PLoS Pathog., 10."
},
{
"DOI": "10.1021/acsinfecdis.9b00296",
"article-title": "Calcium Ions Directly Interact with the Ebola Virus Fusion Peptide To Promote Structure-Function Changes That Enhance Infection",
"author": "Nathan",
"doi-asserted-by": "crossref",
"first-page": "250",
"journal-title": "ACS Infect. Dis.",
"key": "ref_180",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1126/science.1258758",
"article-title": "Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment",
"author": "Sakurai",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "Science",
"key": "ref_181",
"volume": "347",
"year": "2015"
},
{
"DOI": "10.1016/j.jmb.2017.10.017",
"article-title": "The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "3875",
"journal-title": "J. Mol. Biol.",
"key": "ref_182",
"volume": "429",
"year": "2017"
},
{
"DOI": "10.1128/JVI.00426-20",
"article-title": "Ca(2+) Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity",
"author": "Straus",
"doi-asserted-by": "crossref",
"first-page": "e00426-20",
"journal-title": "J. Virol.",
"key": "ref_183",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1021/acsinfecdis.1c00023",
"article-title": "Inhibitors of L-Type Calcium Channels Show Therapeutic Potential for Treating SARS-CoV-2 Infections by Preventing Virus Entry and Spread",
"author": "Straus",
"doi-asserted-by": "crossref",
"first-page": "2807",
"journal-title": "ACS Infect. Dis.",
"key": "ref_184",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2022.110694",
"article-title": "Dynamic Ca(2+) sensitivity stimulates the evolved SARS-CoV-2 spike strain-mediated membrane fusion for enhanced entry",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "110694",
"journal-title": "Cell. Rep.",
"key": "ref_185",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1007/s13238-020-00806-7",
"article-title": "Pan-coronavirus fusion inhibitors as the hope for today and tomorrow",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "84",
"journal-title": "Protein Cell",
"key": "ref_186",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.aav4580",
"article-title": "A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "eaav4580",
"journal-title": "Sci. Adv.",
"key": "ref_187",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.1038/s41423-020-0374-2",
"article-title": "Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "765",
"journal-title": "Cell. Mol. Immunol.",
"key": "ref_188",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3390/v14030597",
"doi-asserted-by": "crossref",
"key": "ref_189",
"unstructured": "Xing, L., Xu, X., Xu, W., Liu, Z., Shen, X., Zhou, J., Xu, L., Pu, J., Yang, C., and Huang, Y. (2022). A Five-Helix-Based SARS-CoV-2 Fusion Inhibitor Targeting Heptad Repeat 2 Domain against SARS-CoV-2 and Its Variants of Concern. Viruses, 14."
},
{
"DOI": "10.1038/s41392-021-00698-x",
"article-title": "SARS-CoV-2-derived fusion inhibitor lipopeptides exhibit highly potent and broad-spectrum activity against divergent human coronaviruses",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "294",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_190",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.2174/0929867326666190805151654",
"article-title": "Antiviral Activities of Human Host Defense Peptides",
"author": "Brice",
"doi-asserted-by": "crossref",
"first-page": "1420",
"journal-title": "Curr. Med. Chem.",
"key": "ref_191",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2022.2051753",
"article-title": "Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "926",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_192",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/ncomms4067",
"article-title": "Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "3067",
"journal-title": "Nat. Commun.",
"key": "ref_193",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1093/pnasnexus/pgac198",
"doi-asserted-by": "crossref",
"key": "ref_194",
"unstructured": "Jana, I.D., Bhattacharya, P., Mayilsamy, K., Banerjee, S., Bhattacharje, G., Das, S., Aditya, S., Ghosh, A., McGill, A.R., and Srikrishnan, S. (2022). Targeting an evolutionarily conserved “E-L-L” motif in the spike protein to develop a small molecule fusion inhibitor against SARS-CoV-2. bioRxiv, pgac198."
},
{
"DOI": "10.3390/v13040651",
"doi-asserted-by": "crossref",
"key": "ref_195",
"unstructured": "Ko, M., Chang, S.Y., Byun, S.Y., Ianevski, A., Choi, I., Pham Hung d’Alexandry d’Orengiani, A.L., Ravlo, E., Wang, W., Bjoras, M., and Kainov, D.E. (2021). Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19. Viruses, 13."
},
{
"DOI": "10.1128/AAC.00399-20",
"article-title": "Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "e00399-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_196",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03431-4",
"article-title": "Clofazimine broadly inhibits coronaviruses including SARS-CoV-2",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "418",
"journal-title": "Nature",
"key": "ref_197",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.5588/ijtld.12.0144",
"article-title": "Systematic review of clofazimine for the treatment of drug-resistant tuberculosis",
"author": "Gopal",
"doi-asserted-by": "crossref",
"first-page": "1001",
"journal-title": "Int. J. Tuberc. Lung Dis.",
"key": "ref_198",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.1038/s41586-020-2577-1",
"article-title": "Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing",
"author": "Riva",
"doi-asserted-by": "crossref",
"first-page": "113",
"journal-title": "Nature",
"key": "ref_199",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1128/AAC.03036-14",
"article-title": "Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection",
"author": "Dyall",
"doi-asserted-by": "crossref",
"first-page": "4885",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_200",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1016/j.celrep.2021.108959",
"article-title": "Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-CoV-2",
"author": "Dittmar",
"doi-asserted-by": "crossref",
"first-page": "108959",
"journal-title": "Cell. Rep.",
"key": "ref_201",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1128/JVI.01441-18",
"article-title": "Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "e01441-18",
"journal-title": "J. Virol.",
"key": "ref_202",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.1016/j.bbamcr.2013.04.011",
"article-title": "Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: Differences between primary and cancer cells",
"author": "Jangamreddy",
"doi-asserted-by": "crossref",
"first-page": "2057",
"journal-title": "Biochim. Biophys. Acta",
"key": "ref_203",
"volume": "1833",
"year": "2013"
},
{
"DOI": "10.1186/s13046-018-0680-z",
"article-title": "Salinomycin, as an autophagy modulator-- a new avenue to anticancer: A review",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "J. Exp. Clin. Cancer Res.",
"key": "ref_204",
"volume": "37",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0044132",
"doi-asserted-by": "crossref",
"key": "ref_205",
"unstructured": "Verdoodt, B., Vogt, M., Schmitz, I., Liffers, S.T., Tannapfel, A., and Mirmohammadsadegh, A. (2012). Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species. PLoS ONE, 7."
},
{
"DOI": "10.3389/fgene.2022.866474",
"article-title": "Anti-Fungal Drug Anidulafungin Inhibits SARS-CoV-2 Spike-Induced Syncytia Formation by Targeting ACE2-Spike Protein Interaction",
"author": "Ahamad",
"doi-asserted-by": "crossref",
"first-page": "866474",
"journal-title": "Front. Genet.",
"key": "ref_206",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1371/journal.pntd.0010363",
"doi-asserted-by": "crossref",
"key": "ref_207",
"unstructured": "Zhang, Z.R., Zhang, Y.N., Zhang, H.Q., Zhang, Q.Y., Li, N., Li, Q., Deng, C.L., Zhang, B., Li, X.D., and Ye, H.Q. (2022). Berbamine hydrochloride potently inhibits SARS-CoV-2 infection by blocking S protein-mediated membrane fusion. PLoS Negl. Trop. Dis., 16."
},
{
"DOI": "10.1038/s41422-020-0305-x",
"article-title": "Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "343",
"journal-title": "Cell. Res.",
"key": "ref_208",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25985",
"article-title": "The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections",
"author": "Musarrat",
"doi-asserted-by": "crossref",
"first-page": "2087",
"journal-title": "J. Med. Virol.",
"key": "ref_209",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1358/dot.2013.49.4.1947288",
"article-title": "Cobicistat, a pharmacoenhancer for HIV treatments",
"author": "Temesgen",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "Drugs Today",
"key": "ref_210",
"volume": "49",
"year": "2013"
},
{
"DOI": "10.1128/mbio.03705-21",
"doi-asserted-by": "crossref",
"key": "ref_211",
"unstructured": "Shytaj, I.L., Fares, M., Gallucci, L., Lucic, B., Tolba, M.M., Zimmermann, L., Adler, J.M., Xing, N., Bushe, J., and Gruber, A.D. (2022). The FDA-Approved Drug Cobicistat Synergizes with Remdesivir To Inhibit SARS-CoV-2 Replication In Vitro and Decreases Viral Titers and Disease Progression in Syrian Hamsters. mBio, 13."
},
{
"DOI": "10.1093/bmb/61.1.13",
"article-title": "The pathogenesis of respiratory syncytial virus disease in childhood",
"author": "McNamara",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Br. Med. Bull.",
"key": "ref_212",
"volume": "61",
"year": "2002"
},
{
"DOI": "10.7326/0003-4819-118-9-199305010-00004",
"article-title": "Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS",
"author": "Koot",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Ann. Intern. Med.",
"key": "ref_213",
"volume": "118",
"year": "1993"
},
{
"DOI": "10.4049/jimmunol.158.8.3996",
"article-title": "HIV-induced T cell syncytia are self-perpetuating and the primary cause of T cell death in culture",
"author": "Sylwester",
"doi-asserted-by": "crossref",
"first-page": "3996",
"journal-title": "J. Immunol.",
"key": "ref_214",
"volume": "158",
"year": "1997"
},
{
"DOI": "10.1007/s00428-021-03053-1",
"article-title": "The pulmonary pathology of COVID-19",
"author": "Bosmuller",
"doi-asserted-by": "crossref",
"first-page": "137",
"journal-title": "Virchows. Arch.",
"key": "ref_215",
"volume": "478",
"year": "2021"
},
{
"DOI": "10.1038/s41418-021-00795-y",
"article-title": "Syncytia formation during SARS-CoV-2 lung infection: A disastrous unity to eliminate lymphocytes",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "2019",
"journal-title": "Cell. Death Differ.",
"key": "ref_216",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.735922",
"article-title": "COVID-19 Lung Pathogenesis in SARS-CoV-2 Autopsy Cases",
"author": "Valdebenito",
"doi-asserted-by": "crossref",
"first-page": "735922",
"journal-title": "Front. Immunol.",
"key": "ref_217",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1128/JVI.02699-09",
"article-title": "Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "8132",
"journal-title": "J. Virol.",
"key": "ref_218",
"volume": "84",
"year": "2010"
},
{
"DOI": "10.1016/j.cell.2021.05.005",
"article-title": "Tackling COVID-19 with neutralizing monoclonal antibodies",
"author": "Corti",
"doi-asserted-by": "crossref",
"first-page": "3086",
"journal-title": "Cell",
"key": "ref_219",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1146/annurev-med-042420-113838",
"article-title": "SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Annu. Rev. Med.",
"key": "ref_220",
"volume": "73",
"year": "2022"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"article-title": "Neutralizing monoclonal antibodies for treatment of COVID-19",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "382",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_221",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.04.033",
"article-title": "Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia",
"author": "Asarnow",
"doi-asserted-by": "crossref",
"first-page": "3192",
"journal-title": "Cell",
"key": "ref_222",
"volume": "184",
"year": "2021"
}
],
"reference-count": 222,
"references-count": 222,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/12/18/6079"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication",
"type": "journal-article",
"volume": "12"
}