Niclosamide: a potential treatment option for COVID-19
et al., International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850, Jan 2023
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
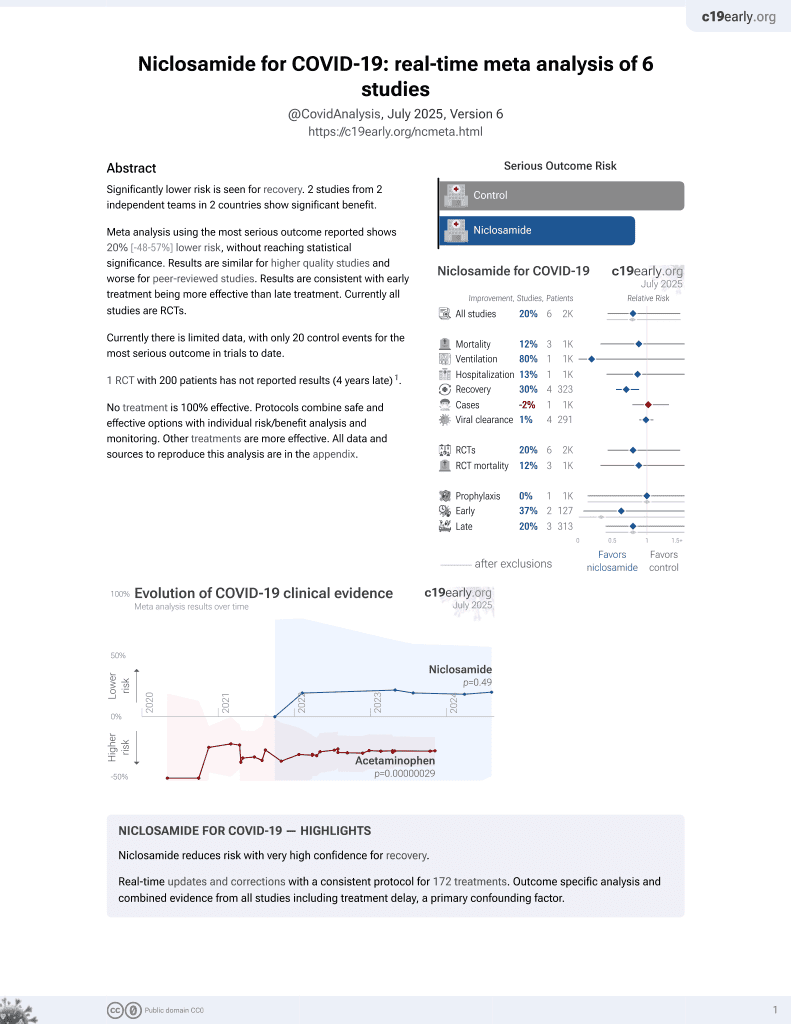
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of niclosamide as a potential treatment option for COVID-19. Authors report antiviral activity against SARS-CoV-2 in vitro, with an IC50 of 0.28 μM. However, the currently available oral formulations limit systemic levels. To improve bioavailability, various formulation strategies have been explored for oral, inhalation, and intranasal delivery. Potential antiviral mechanisms include blocking viral endocytosis and inhibiting autophagy.
1.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
2.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
3.
Ali et al., SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication, Journal of Clinical Medicine, doi:10.3390/jcm12186079.
4.
Vibhute et al., Niclosamide: a potential treatment option for COVID-19, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850.
5.
Abed et al., Evaluation the plausibility of repurpose of levamisole and niclosamide in treatment of covid-19, Journal of Pharmaceutical Technolgy, doi:10.37662/jpt.2022.999481.
6.
Cesar-Silva et al., The Endolysosomal System: The Acid Test for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23094576.
Vibhute et al., 7 Jan 2023, peer-reviewed, 5 authors.
Contact: vibhuteshweta5@gmail.com.
NICLOSAMIDE: A POTENTIAL TREATMENT OPTION FOR COVID-19
International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2023v15i1.45850
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a global health hazard due to its rapid dissemination and limited treatment options. Identification of possible treatments that may kill the virus, speed up the recovery, or reduce the case fatality rate is a need of hour. However, developing and producing particular COVID-19 medicines and vaccines is a time-consuming process with possibilities of clinical failures due to safety or efficacy issue. Medication repositioning is a safer and quicker approach for dealing with the COVID-19 worldwide threat right now. Out of 48 FDA-approved medicines tested against SARS-CoV-2, niclosamide is one amongst few that has shown potential in vitro antiviral activity against SARS-CoV-2. However, the currently available oral conventional formulation of niclosamide results in systemic medication levels those are unsatisfactory to inhibit SARS-CoV-2. Hence, various formulation strategies have been adapted in order to achieve an optimum therapeutic outcome of niclosamide when delivered via oral, inhalation, and intranasal routes. Some of these formulations are presently undergoing clinical trials. The current review focuses on the mechanisms of action of niclosamide and its repurposing effectiveness against COVID-19. The delivery strategies to improve its bioavailability have been overviewed. The recently completed and ongoing clinical trials have also been summarized.
CONFLICTS OF INTERESTS
Declared none
References
Ai, Wood, Yang, Welsh, Niclosamide is a negative allosteric modulator of Group I metabotropic glutamate receptors: implications for neuropathic pain, Pharm Res, doi:10.1007/s11095-016-2027-9
Andrews, Thyssen, Lorke, The biology and toxicology of molluscicides, bayluscide, Pharmacol Ther, doi:10.1016/0163-7258(82)90064-x
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther, doi:10.1002/cpt.1909
Barbosa, Lobenberg, De Araujo, Bou-Chacra, Niclosamide repositioning for treating cancer: challenges and nano-based drug delivery opportunities, Eur J Pharm Biopharm, doi:10.1016/j.ejpb.2019.05.004
Biju, Kp, Revadigar, Dsouza, Iqbal et al., A review on the impact of the Covid-19 pandemic on the health care sector, Int J Pharm Pharm Sci, doi:10.22159/ijpps.2021v13i10.42566
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patientadaptable treatment for coronavirus infections and sequalae, PLOS ONE, doi:10.1371/journal.pone.0246803
Chen, Mook, Premont, Wang, Niclosamide: beyond an antihelminthic drug, Cell Signal, doi:10.1016/j.cellsig.2017.04.001
Choi, Piao, Rejinold, Yu, Kim et al., Hydrotalcite-niclosamide nanohybrid as oral formulation towards SARS-CoV-2 viral infections, Pharmaceuticals, doi:10.3390/ph14050486
Chu, Chan, Wang, Yuen, Chai et al., Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19, Clinical Infectious Diseases, doi:10.1093/cid/ciaa410
Costabile, Angelo, Rampioni, Bondì, Pompili et al., Toward repositioning niclosamide for antivirulence therapy of Pseudomonas aeruginosa Lung infections: development of inhalable formulations through nanosuspension technology, Mol Pharm, doi:10.1021/acs.molpharmaceut.5b00098
Damiani, Fiorentino, Palma, Foschini, Lazzarotto et al., Pathological post-mortem findings in lungs infected with SARS-CoV-2, J Pathol, doi:10.1002/path.5549
Eduardo, Contribution to the improvement of an oral formulation of niclosamide, an antihelmintic drug candidate for repurposing in SARS-CoV-2 and other viruses, doi:10.26434/chemrxiv.12056187.v1
Gassen, Niemeyer, Muth, Martinelli, Gassen, SKP2 attenuates autophagy through Beclin1ubiquitination and its inhibition reduces MERS-coronavirus infection, Nat Commun, doi:10.1038/s41467-019-13659-4
Gassen, Papies, Bajaj, Dethloff, Emanuel et al., Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics, Microbiology, doi:10.1101/2020.04.15.997254
Gil, Ginex, Maestro, Nozal, Gil et al., Covid-19: drug targets and potential treatments, J Med Chem, doi:10.1021/acs.jmedchem.0c00606
Grifasi, Chierotti, Gaglioti, Gobetto, Maini et al., Using salt cocrystals to improve the solubility of niclosamide, Cryst Growth Des, doi:10.1021/acs.cgd.5b00106
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Gui, Song, Zhou, Xu, Chen et al., Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding, Cell Res, doi:10.1038/cr.2016.152
Gwisai, Hollingsworth, Cowles, Tharmalingam, Mylonakis et al., Repurposing niclosamide as a versatile antimicrobial surface coating against device-associated, hospital-acquired bacterial infections, Biomed Mater, doi:10.1088/1748-605X/aa7105
Hobson, Savage, Dwyer, Unsworth, Massam et al., Scalable nanoprecipitation of niclosamide and in vivo demonstration of long-acting delivery after intramuscular injection, Nanoscale, doi:10.1039/d1nr00309g
Jara, Warnken, Sahakijpijarn, Moon, Maier et al., Niclosamide inhalation powder made by thinfilm freezing: multi-dose tolerability and exposure in rats and pharmacokinetics in hamsters, Int J Pharm, doi:10.1016/j.ijpharm.2021.120701
Jara, Warnken, Williams, Amorphous solid dispersions and the contribution of nanoparticles to in vitro dissolution and in vivo testing: niclosamide as a case study, Pharmaceutics, doi:10.3390/pharmaceutics13010097
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDAapproved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLOS Pathog, doi:10.1371/journal.ppat.1002976
Kadri, Lambourne, Mehellou, Niclosamide, a drug with Many (Re) purposes, Chem Med Chem, doi:10.1002/cmdc.201800100
Kifle, Ayele, Enyew, Drug repurposing approach, potential drugs, and novel drug targets for COVID-19 treatment, J Environ Public Health, doi:10.1155/2021/6631721
Li, Yan, Zhang, Zhen, Liu, Niclosamide ethanolamine inhibits artery constriction, Pharmacol Res, doi:10.1016/j.phrs.2016.11.008
Liang, Huang, Xiao, Zen, Lao et al., Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis, Inflamm Res, doi:10.1007/s00011-015-0801-5
Lodhi, Panwar, Dongre, Review study on analysis of the solubility of biopharmaceutical classification system class ii drugs in a self-emulsifying drug delivery system, Asian J Pharm Clin Res
Nagasree, Santhi, Kumar, Jyothi, Lakshmi, Review on drug repurposing
Naqvi, Mohiyuddin, Gopinath, Niclosamide loaded biodegradable chitosan nanocargoes: an in vitro study for potential application in cancer therapy, R Soc Open Sci, doi:10.1098/rsos.170611
Niharika, Niharika, Aishwarya, Nikitha, Butool et al., Coronavirus-a virus in learning, Int J Curr Pharm Sci, doi:10.22159/ijcpr.2020v12i4.39078
Parmar, Panchal, Drug reposition of non-cancer drugs for cancer treatments via pharmacovigilance approachrepurposing drugs in oncology, Asian J Pharm Clin Res, doi:10.22159/ajpcr.2019.v12i2.29523
Pearson, Hewlett, Niclosamide therapy for tapeworm infections, Ann Intern Med, doi:10.7326/0003-4819-102-4-550
Petersen, Koopmans, Go, Hamer, Petrosillo et al., Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30484-9
Pindiprolu, Pindiprolu, Plausible mechanisms of niclosamide as an antiviral agent against COVID-19, Med Hypotheses, doi:10.1016/j.mehy.2020.109765
Prather, Maclean, Shi, Boadu, Paquet et al., Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model, Biol Reprod, doi:10.1095/biolreprod.116.140236
Rehman, Khan, Khan, Shafique, Khan, Fabrication of niclosamide loaded solid lipid nanoparticles: in vitro characterization and comparative in vivo evaluation, Artif Cells Nanomed Biotechnol, doi:10.1080/21691401.2017.1396996
Rejinold, Choi, Piao, Choy, Bovine serum albumincoated niclosamide-Zein nanoparticles as potential injectable medicine against COVID-19, Materials, doi:10.3390/ma14143792
Russo, Pellosi, Pagliara, Milone, Pucci et al., Biotin-targeted pluronic(®) P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drugresistant lung cancer cells, Int J Pharm, doi:10.1016/j.ijpharm.2016.06.118
Sanphui, Kumar, Nangia, Pharmaceutical cocrystals of niclosamide, Cryst Growth Des, doi:10.1021/cg300784v
Singh, Parida, Lingaraju, Kesavan, Kumar et al., Drug repurposing approach to fight COVID-19, Pharmacol Rep, doi:10.1007/s43440-020-00155-6
Sp, Insights into corona/coronavirus disease 2019 pandemic-opinion versus evidence, Asian J Pharm Clin Res
Tao, Zhang, Zeng, Shulman, Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice, Nat Med, doi:10.1038/nm.3699
Thomson, COVID-19: social distancing, ACE 2 receptors, protease inhibitors and beyond?, Int J Clin Pract, doi:10.1111/ijcp.13503
Tojo, Vascular endothelial glycocalyx damage in COVID-19, Int J Mol Sci, doi:10.3390/ijms21249712
Velavan, Meyer, The COVID-19 epidemic, Trop Med Int Health, doi:10.1111/tmi.13383
Wang, Gaikwad, Mccarthy, Gonzalez-Juarrero, Li et al., Lipid nanoparticle formulation of niclosamide (Nano NCM) effectively inhibits SARS-CoV-2 replication in vitro, Precis Nanomed, doi:10.33218/001c.18813
Xie, Yao, Octenylsuccinate hydroxypropyl phytoglycogen enhances the solubility and in vitro antitumor efficacy of niclosamide, Int J Pharm, doi:10.1016/j.ijpharm.2017.11.004
Xu, Shi, Li, Zhou, Broad spectrum antiviral agent niclosamide and its therapeutic potential, ACS Infect Dis, doi:10.1021/acsinfecdis.0c00052
Ye, Zhang, Zhang, Wang, Wu, Design and evaluation of injectable niclosamide nanocrystals prepared by wet media milling technique, Drug Dev Ind Pharm, doi:10.3109/03639045.2014.954585
Yu, Piao, Rejinold, Choi, Choy, Niclosamide-clay intercalates coated with nonionic polymer for enhanced bioavailability toward COVID-19 treatment, Polymers, doi:10.3390/polym13071044
Zeyada, Rahman, El-Karef, Yahia, El-Sherbiny et al., Niclosamide-loaded polymeric micelles ameliorate hepatocellular carcinoma in vivo through targeting Wnt and Notch pathways, Life Sci, doi:10.1016/j.lfs.2020.118458
Zhang, Zhang, Zhang, Zhang, Wu, Significantly enhanced bioavailability of niclosamide through submicron lipid emulsions with or without PEG-lipid: a comparative study, J Microencapsul, doi:10.3109/02652048.2015.1057251
DOI record:
{
"DOI": "10.22159/ijap.2023v15i1.45850",
"ISSN": [
"0975-7058"
],
"URL": "http://dx.doi.org/10.22159/ijap.2023v15i1.45850",
"abstract": "<jats:p>Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a global health hazard due to its rapid dissemination and limited treatment options. Identification of possible treatments that may kill the virus, speed up the recovery, or reduce the case fatality rate is a need of hour. However, developing and producing particular COVID-19 medicines and vaccines is a time-consuming process with possibilities of clinical failures due to safety or efficacy issue. Medication repositioning is a safer and quicker approach for dealing with the COVID-19 worldwide threat right now. Out of 48 FDA-approved medicines tested against SARS-CoV-2, niclosamide is one amongst few that has shown potential in vitro antiviral activity against SARS-CoV-2. However, the currently available oral conventional formulation of niclosamide results in systemic medication levels those are unsatisfactory to inhibit SARS-CoV-2. Hence, various formulation strategies have been adapted in order to achieve an optimum therapeutic outcome of niclosamide when delivered via oral, inhalation, and intranasal routes. Some of these formulations are presently undergoing clinical trials. The current review focuses on the mechanisms of action of niclosamide and its repurposing effectiveness against COVID-19. The delivery strategies to improve its bioavailability have been overviewed. The recently completed and ongoing clinical trials have also been summarized.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7809-9958",
"affiliation": [],
"authenticated-orcid": false,
"family": "VIBHUTE",
"given": "SHWETA",
"sequence": "first"
},
{
"affiliation": [],
"family": "KASAR",
"given": "ADITI",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7672-6823",
"affiliation": [],
"authenticated-orcid": false,
"family": "MAHALE",
"given": "HRISHIKESH",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GAIKWAD",
"given": "MAHESH",
"sequence": "additional"
},
{
"affiliation": [],
"family": "KULKARNI",
"given": "MADHUR",
"sequence": "additional"
}
],
"container-title": "International Journal of Applied Pharmaceutics",
"container-title-short": "Int J App Pharm",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
11
]
],
"date-time": "2023-01-11T07:42:09Z",
"timestamp": 1673422929000
},
"deposited": {
"date-parts": [
[
2023,
1,
11
]
],
"date-time": "2023-01-11T07:59:59Z",
"timestamp": 1673423999000
},
"indexed": {
"date-parts": [
[
2024,
6,
20
]
],
"date-time": "2024-06-20T21:58:16Z",
"timestamp": 1718920696278
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2023,
1,
7
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
7
]
],
"date-time": "2023-01-07T00:00:00Z",
"timestamp": 1673049600000
}
}
],
"link": [
{
"URL": "https://innovareacademics.in/journals/index.php/ijap/article/download/45850/27480",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://innovareacademics.in/journals/index.php/ijap/article/download/45850/27522",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://innovareacademics.in/journals/index.php/ijap/article/download/45850/27480",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9422",
"original-title": [],
"page": "50-56",
"prefix": "10.22159",
"published": {
"date-parts": [
[
2023,
1,
7
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
7
]
]
},
"publisher": "Innovare Academic Sciences Pvt Ltd",
"reference": [
{
"DOI": "10.1021/acs.jmedchem.0c00606",
"doi-asserted-by": "crossref",
"key": "1055339",
"unstructured": "Gil C, Ginex T, Maestro I, Nozal V, Barrado Gil L, Cuesta Geijo MA. Covid-19: drug targets and potential treatments. J Med Chem. 2020 Nov 12;63(21):12359-86. doi: 10.1021/ acs.jmedchem.0c00606, PMID 32511912."
},
{
"DOI": "10.22159/ijpps.2021v13i10.42566",
"doi-asserted-by": "crossref",
"key": "1055340",
"unstructured": "Biju P, KP, Revadigar V, Dsouza S, Asif Iqbal M, Ahmed G. A review on the impact of the Covid-19 pandemic on the health care sector. Int J Pharm Pharm Sci. 2021 Oct 1:1-6. doi: 10.22159/ijpps.2021v13i10.42566."
},
{
"DOI": "10.1016/S1473-3099(20)30484-9",
"doi-asserted-by": "crossref",
"key": "1055341",
"unstructured": "Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020 Sep;20(9):e238-44. doi: 10.1016/S1473-3099(20)30484-9, PMID 32628905."
},
{
"DOI": "10.1007/s43440-020-00155-6",
"doi-asserted-by": "crossref",
"key": "1055342",
"unstructured": "Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID-19. Pharmacol Rep. 2020 Dec;72(6):1479-508. doi: 10.1007/s43440-020-00155-6, PMID 32889701."
},
{
"DOI": "10.22159/ijcpr.2020v12i4.39078",
"doi-asserted-by": "crossref",
"key": "1055343",
"unstructured": "Niharika D, Niharika B, Aishwarya T, Nikitha A, Butool R, Ibrahim M. Coronavirus-a virus in learning. Int J Curr Pharm Sci. 2020 Jul 16:7-10. doi: 10.22159/ijcpr.2020v12i4.39078."
},
{
"DOI": "10.1111/tmi.13383",
"doi-asserted-by": "crossref",
"key": "1055344",
"unstructured": "Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020 Mar;25(3):278-80. doi: 10.1111/tmi.13383, PMID 32052514."
},
{
"DOI": "10.22159/ajpcr.2021.v14i7.41754",
"doi-asserted-by": "crossref",
"key": "1055345",
"unstructured": "SP, Sundararajan M, AM. Insights into corona/coronavirus disease 2019 pandemic–opinion versus evidence. Asian J Pharm Clin Res. 2021 May 28:13-5."
},
{
"DOI": "10.1155/2021/6631721",
"doi-asserted-by": "crossref",
"key": "1055346",
"unstructured": "Kifle ZD, Ayele AG, Enyew EF. Drug repurposing approach, potential drugs, and novel drug targets for COVID-19 treatment. J Environ Public Health. 2021;2021:6631721. doi: 10.1155/2021/6631721, PMID 33953756."
},
{
"DOI": "10.1016/j.ijpharm.2016.06.118",
"doi-asserted-by": "crossref",
"key": "1055347",
"unstructured": "Russo A, Pellosi DS, Pagliara V, Milone MR, Pucci B, Caetano W. Biotin-targeted pluronic(®) P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int J Pharm. 2016 Sep 10;511(1):127-39. doi: 10.1016/j.ijpharm.2016.06.118, PMID 27374195."
},
{
"DOI": "10.1128/AAC.00819-20",
"doi-asserted-by": "crossref",
"key": "1055348",
"unstructured": "Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020 Jun 23;64(7):e00819-20. doi: 10.1128/AAC.00819-20, PMID 32366720."
},
{
"DOI": "10.1021/acsinfecdis.0c00052",
"doi-asserted-by": "crossref",
"key": "1055349",
"unstructured": "Xu J, Shi PY, Li H, Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020 May 8;6(5):909-15. doi: 10.1021/acsinfecdis.0c00052, PMID 32125140."
},
{
"key": "1055350",
"unstructured": "Nagasree KP, Santhi K, Kumar RS, Jyothi BA, Lakshmi VJ. Review on drug repurposing. 2020;7(10):16."
},
{
"DOI": "10.22159/ajpcr.2019.v12i2.29523",
"doi-asserted-by": "crossref",
"key": "1055351",
"unstructured": "Parmar MB, Panchal S. Drug reposition of non-cancer drugs for cancer treatments via pharmacovigilance approach–repurposing drugs in oncology. Asian J Pharm Clin Res. 2019 Jan 31:310-4. doi: 10.22159/ajpcr.2019.v12i2.29523."
},
{
"DOI": "10.1016/0163-7258(82)90064-X",
"doi-asserted-by": "crossref",
"key": "1055352",
"unstructured": "Andrews P, Thyssen J, Lorke D. The biology and toxicology of molluscicides, bayluscide. Pharmacol Ther. 1982;19(2):245-95. doi: 10.1016/0163-7258(82)90064-x, PMID 6763710."
},
{
"DOI": "10.7326/0003-4819-102-4-550",
"doi-asserted-by": "crossref",
"key": "1055353",
"unstructured": "Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Ann Intern Med. 1985 Apr;102(4):550-1. doi: 10.7326/0003-4819-102-4-550, PMID 3977200."
},
{
"key": "1055354",
"unstructured": "World Health Organization model list of essential medicines; 2019."
},
{
"DOI": "10.1016/j.cellsig.2017.04.001",
"doi-asserted-by": "crossref",
"key": "1055355",
"unstructured": "Chen W, Mook RA, Premont RT, Wang J. Niclosamide: beyond an antihelminthic drug. Cell Signal. 2018 Jan;41:89-96. doi: 10.1016/j.cellsig.2017.04.001, PMID 28389414."
},
{
"DOI": "10.1002/cmdc.201800100",
"doi-asserted-by": "crossref",
"key": "1055356",
"unstructured": "Kadri H, Lambourne OA, Mehellou Y. Niclosamide, a drug with Many (Re) purposes. Chem Med Chem. 2018 Jun 6;13(11):1088-91. doi: 10.1002/cmdc.201800100, PMID 29603892."
},
{
"DOI": "10.1007/s11095-016-2027-9",
"doi-asserted-by": "crossref",
"key": "1055357",
"unstructured": "Ai N, Wood RD, Yang E, Welsh WJ. Niclosamide is a negative allosteric modulator of Group I metabotropic glutamate receptors: implications for neuropathic pain. Pharm Res. 2016 Dec;33(12):3044-56. doi: 10.1007/s11095-016-2027-9, PMID 27631130."
},
{
"DOI": "10.1016/j.ejpb.2019.05.004",
"doi-asserted-by": "crossref",
"key": "1055358",
"unstructured": "Barbosa EJ, Lobenberg R, de Araujo GLB, Bou-Chacra NA. Niclosamide repositioning for treating cancer: challenges and nano-based drug delivery opportunities. Eur J Pharm Biopharm. 2019 Aug;141:58-69. doi: 10.1016/j.ejpb.2019.05.004, PMID 31078739."
},
{
"DOI": "10.1016/j.phrs.2016.11.008",
"doi-asserted-by": "crossref",
"key": "1055359",
"unstructured": "Li SL, Yan J, Zhang YQ, Zhen CL, Liu MY, Jin J. Niclosamide ethanolamine inhibits artery constriction. Pharmacol Res. 2017 Jan;115:78-86. doi: 10.1016/j.phrs.2016.11.008, PMID 27872020."
},
{
"DOI": "10.1007/s00011-015-0801-5",
"doi-asserted-by": "crossref",
"key": "1055360",
"unstructured": "Liang L, Huang M, Xiao Y, Zen S, Lao M, Zou Y. Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm Res. 2015 Apr;64(3-4):225-33. doi: 10.1007/s00011-015-0801-5, PMID 25708600."
},
{
"DOI": "10.1095/biolreprod.116.140236",
"doi-asserted-by": "crossref",
"key": "1055361",
"unstructured": "Prather GR, MacLean JA, Shi M, Boadu DK, Paquet M, Hayashi K. Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model. Biol Reprod. 2016 Oct 1;95(4):76. doi: 10.1095/biolreprod.116.140236, PMID 27535961."
},
{
"DOI": "10.1038/nm.3699",
"doi-asserted-by": "crossref",
"key": "1055362",
"unstructured": "Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med. 2014 Nov;20(11):1263-9. doi: 10.1038/nm.3699, PMID 25282357."
},
{
"DOI": "10.1088/1748-605X/aa7105",
"doi-asserted-by": "crossref",
"key": "1055363",
"unstructured": "Gwisai T, Hollingsworth NR, Cowles S, Tharmalingam N, Mylonakis E, Fuchs BB. Repurposing niclosamide as a versatile antimicrobial surface coating against device-associated, hospital-acquired bacterial infections. Biomed Mater. 2017 Jul 12;12(4):045010. doi: 10.1088/1748-605X/aa7105, PMID 28471351."
},
{
"DOI": "10.1038/s41467-019-13659-4",
"doi-asserted-by": "crossref",
"key": "1055364",
"unstructured": "Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun. 2019 Dec 18;10(1):5770. doi: 10.1038/s41467-019-13659-4, PMID 31852899."
},
{
"DOI": "10.1002/cpt.1909",
"doi-asserted-by": "crossref",
"key": "1055365",
"unstructured": "Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, Curley P. Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020 Oct;108(4):775-90. doi: 10.1002/cpt.1909, PMID 32438446."
},
{
"DOI": "10.1038/cr.2016.152",
"doi-asserted-by": "crossref",
"key": "1055366",
"unstructured": "Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017 Jan;27(1):119-29. doi: 10.1038/cr.2016.152, PMID 28008928."
},
{
"DOI": "10.1111/ijcp.13503",
"doi-asserted-by": "crossref",
"key": "1055367",
"unstructured": "Thomson G. COVID-19: social distancing, ACE 2 receptors, protease inhibitors and beyond? Int J Clin Pract. 2020 Jul;74(7):e13503. doi: 10.1111/ijcp.13503, PMID 32187421."
},
{
"DOI": "10.1371/journal.ppat.1002976",
"doi-asserted-by": "crossref",
"key": "1055368",
"unstructured": "Jurgeit A, McDowell R, Moese S, Meldrum E, Schwendener R, Greber UF. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLOS Pathog. 2012;8(10):e1002976. doi: 10.1371/journal.ppat.1002976, PMID 23133371."
},
{
"DOI": "10.1016/j.mehy.2020.109765",
"doi-asserted-by": "crossref",
"key": "1055369",
"unstructured": "Pindiprolu SKSS, Pindiprolu SH. Plausible mechanisms of niclosamide as an antiviral agent against COVID-19. Med Hypotheses. 2020 Apr 22;140:109765. doi: 10.1016/j.mehy.2020.109765, PMID 32361588."
},
{
"DOI": "10.1101/2020.04.15.997254",
"doi-asserted-by": "crossref",
"key": "1055370",
"unstructured": "Gassen NC, Papies J, Bajaj T, Dethloff F, Emanuel J, Weckmann K. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. Microbiology. 2020. doi: 10.1101/2020.04.15.997254."
},
{
"DOI": "10.22159/ajpcr.2022.v15i2.43303",
"doi-asserted-by": "crossref",
"key": "1055371",
"unstructured": "Lodhi DS, Panwar AS, Dongre N. Review study on analysis of the solubility of biopharmaceutical classification system class ii drugs in a self-emulsifying drug delivery system. Asian J Pharm Clin Res. 2021; Dec 16:36-45."
},
{
"DOI": "10.26434/chemrxiv.12056187.v1",
"doi-asserted-by": "crossref",
"key": "1055372",
"unstructured": "Eduardo B. Contribution to the improvement of an oral formulation of niclosamide, an antihelmintic drug candidate for repurposing in SARS-CoV-2 and other viruses; 2020. doi: 10.26434/chemrxiv.12056187.v1"
},
{
"DOI": "10.1080/21691401.2017.1396996",
"doi-asserted-by": "crossref",
"key": "1055373",
"unstructured": "Rehman MU, Khan MA, Khan WS, Shafique M, Khan M. Fabrication of niclosamide loaded solid lipid nanoparticles: in vitro characterization and comparative in vivo evaluation. Artif Cells Nanomed Biotechnol. 2018 Dec;46(8):1926-34. doi: 10.1080/21691401.2017.1396996, PMID 29113501."
},
{
"DOI": "10.1021/acs.cgd.5b00106",
"doi-asserted-by": "crossref",
"key": "1055374",
"unstructured": "Grifasi F, Chierotti MR, Gaglioti K, Gobetto R, Maini L, Braga D. Using salt cocrystals to improve the solubility of niclosamide. Cryst Growth Des. 2015 Apr 1;15(4):1939-48. doi: 10.1021/acs.cgd.5b00106."
},
{
"DOI": "10.1021/cg300784v",
"doi-asserted-by": "crossref",
"key": "1055375",
"unstructured": "Sanphui P, Kumar SS, Nangia A. Pharmaceutical cocrystals of niclosamide. Cryst Growth Des. 2012 Sep 5;12(9):4588-99. doi: 10.1021/cg300784v."
},
{
"DOI": "10.1016/j.ijpharm.2017.11.004",
"doi-asserted-by": "crossref",
"key": "1055376",
"unstructured": "Xie Y, Yao Y. Octenylsuccinate hydroxypropyl phytoglycogen enhances the solubility and in vitro antitumor efficacy of niclosamide. Int J Pharm. 2018 Jan 15;535(1-2):157-63. doi: 10.1016/j.ijpharm.2017.11.004, PMID 29113805."
},
{
"DOI": "10.1016/j.lfs.2020.118458",
"doi-asserted-by": "crossref",
"key": "1055377",
"unstructured": "Zeyada MS, Abdel Rahman N, El-Karef A, Yahia S, El-Sherbiny IM, Eissa LA. Niclosamide-loaded polymeric micelles ameliorate hepatocellular carcinoma in vivo through targeting Wnt and Notch pathways. Life Sci. 2020 Nov 15;261:118458. doi: 10.1016/j.lfs.2020.118458, PMID 32961231."
},
{
"DOI": "10.1021/acs.molpharmaceut.5b00098",
"doi-asserted-by": "crossref",
"key": "1055378",
"unstructured": "Costabile G, d’Angelo I, Rampioni G, Bondì R, Pompili B, Ascenzioni F. Toward repositioning niclosamide for antivirulence therapy of Pseudomonas aeruginosa Lung infections: development of inhalable formulations through nanosuspension technology. Mol Pharm. 2015 Aug 3;12(8):2604-17. doi: 10.1021/acs.molpharmaceut.5b00098, PMID 25974285."
},
{
"DOI": "10.1098/rsos.170611",
"doi-asserted-by": "crossref",
"key": "1055379",
"unstructured": "Naqvi S, Mohiyuddin S, Gopinath P. Niclosamide loaded biodegradable chitosan nanocargoes: an in vitro study for potential application in cancer therapy. R Soc Open Sci. 2017 Nov;4(11):170611. doi: 10.1098/rsos.170611, PMID 29291056."
},
{
"DOI": "10.3109/02652048.2015.1057251",
"doi-asserted-by": "crossref",
"key": "1055380",
"unstructured": "Zhang X, Zhang Y, Zhang T, Zhang J, Wu B. Significantly enhanced bioavailability of niclosamide through submicron lipid emulsions with or without PEG-lipid: a comparative study. J Microencapsul. 2015;32(5):496-502. doi: 10.3109/02652048.2015.1057251, PMID 26079596."
},
{
"DOI": "10.3109/03639045.2014.954585",
"doi-asserted-by": "crossref",
"key": "1055381",
"unstructured": "Ye Y, Zhang X, Zhang T, Wang H, Wu B. Design and evaluation of injectable niclosamide nanocrystals prepared by wet media milling technique. Drug Dev Ind Pharm. 2015;41(9):1416-24. doi: 10.3109/03639045.2014.954585, PMID 25204767."
},
{
"DOI": "10.1039/D1NR00309G",
"doi-asserted-by": "crossref",
"key": "1055382",
"unstructured": "Hobson JJ, Savage AC, Dwyer AB, Unsworth C, Massam J, Arshad U. Scalable nanoprecipitation of niclosamide and in vivo demonstration of long-acting delivery after intramuscular injection. Nanoscale. 2021 Apr 7;13(13):6410-6. doi: 10.1039/d1nr00309g, PMID 33885522."
},
{
"DOI": "10.3390/ph14050486",
"doi-asserted-by": "crossref",
"key": "1055383",
"unstructured": "Choi G, Piao H, Rejinold NS, Yu S, Kim KY, Jin GW. Hydrotalcite-niclosamide nanohybrid as oral formulation towards SARS-CoV-2 viral infections. Pharmaceuticals (Basel). 2021 May 19;14(5):486. doi: 10.3390/ph14050486, PMID 34069716."
},
{
"DOI": "10.3390/polym13071044",
"doi-asserted-by": "crossref",
"key": "1055384",
"unstructured": "Yu S, Piao H, Rejinold NS, Jin G, Choi G, Choy JH. Niclosamide-clay intercalates coated with nonionic polymer for enhanced bioavailability toward COVID-19 treatment. Polymers. 2021 Mar 26;13(7):1044. doi: 10.3390/polym13071044, PMID 33810527."
},
{
"DOI": "10.33218/001c.18813",
"doi-asserted-by": "crossref",
"key": "1055385",
"unstructured": "Wang G, Gaikwad H, McCarthy MK, Gonzalez-Juarrero M, Li Y, Armstrong M. Lipid nanoparticle formulation of niclosamide (Nano NCM) effectively inhibits SARS-CoV-2 replication in vitro. Precis Nanomed. 2021 Apr 17;4(1):724-37. doi: 10.33218/001c.18813, PMID 34676370."
},
{
"DOI": "10.3390/pharmaceutics13010097",
"doi-asserted-by": "crossref",
"key": "1055386",
"unstructured": "Jara MO, Warnken ZN, Williams RO. Amorphous solid dispersions and the contribution of nanoparticles to in vitro dissolution and in vivo testing: niclosamide as a case study. Pharmaceutics. 2021 Jan 14;13(1):97. doi: 10.3390/pharmaceutics13010097, PMID 33466598."
},
{
"DOI": "10.1093/cid/ciaa410",
"doi-asserted-by": "crossref",
"key": "1055387",
"unstructured": "Chu H, Chan JFW, Wang Y, Yuen TTT, Chai Y, Hou Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clinical Infectious Diseases. 2020 Sep 12;71(6):1400-9. doi: 10.1093/cid/ciaa410."
},
{
"DOI": "10.1002/path.5549",
"doi-asserted-by": "crossref",
"key": "1055388",
"unstructured": "Damiani S, Fiorentino M, De Palma A, Foschini MP, Lazzarotto T, Gabrielli L. Pathological post-mortem findings in lungs infected with SARS-CoV-2. J Pathol. 2021 Jan;253(1):31-40. doi: 10.1002/path.5549, PMID 32930394."
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "crossref",
"key": "1055389",
"unstructured": "Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-20. doi: 10.1056/NEJMoa2002032, PMID 32109013."
},
{
"DOI": "10.1371/journal.pone.0246803",
"doi-asserted-by": "crossref",
"key": "1055390",
"unstructured": "Brunaugh AD, Seo H, Warnken Z, Ding L, Seo SH, Smyth HDC. Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae. PLOS ONE. 2021;16(2):e0246803. doi: 10.1371/journal.pone.0246803, PMID 33571320."
},
{
"DOI": "10.1016/j.ijpharm.2021.120701",
"doi-asserted-by": "crossref",
"key": "1055391",
"unstructured": "Jara MO, Warnken ZN, Sahakijpijarn S, Moon C, Maier EY, Christensen DJ. Niclosamide inhalation powder made by thin-film freezing: multi-dose tolerability and exposure in rats and pharmacokinetics in hamsters. Int J Pharm. 2021 Jun 15;603:120701. doi: 10.1016/j.ijpharm.2021.120701, PMID 33989748."
},
{
"DOI": "10.3390/ijms21249712",
"doi-asserted-by": "crossref",
"key": "1055392",
"unstructured": "Yamaoka Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci. 2020 Dec 19;21(24):E9712. doi: 10.3390/ijms21249712, PMID 33352699."
},
{
"DOI": "10.3390/ma14143792",
"doi-asserted-by": "crossref",
"key": "1055393",
"unstructured": "Rejinold NS, Choi G, Piao H, Choy JH. Bovine serum albumin-coated niclosamide-Zein nanoparticles as potential injectable medicine against COVID-19. Materials. 2021 Jul 7;14(14):3792. doi: 10.3390/ma14143792."
}
],
"reference-count": 55,
"references-count": 55,
"relation": {},
"resource": {
"primary": {
"URL": "https://innovareacademics.in/journals/index.php/ijap/article/view/45850"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "NICLOSAMIDE: A POTENTIAL TREATMENT OPTION FOR COVID-19",
"type": "journal-article"
}