A Phase 2/3 Randomized and Placebo-Controlled Study of ANA001 in Moderate and Severe COVID-19 Patients
et al., NCT04603924, NCT04603924, Aug 2024
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
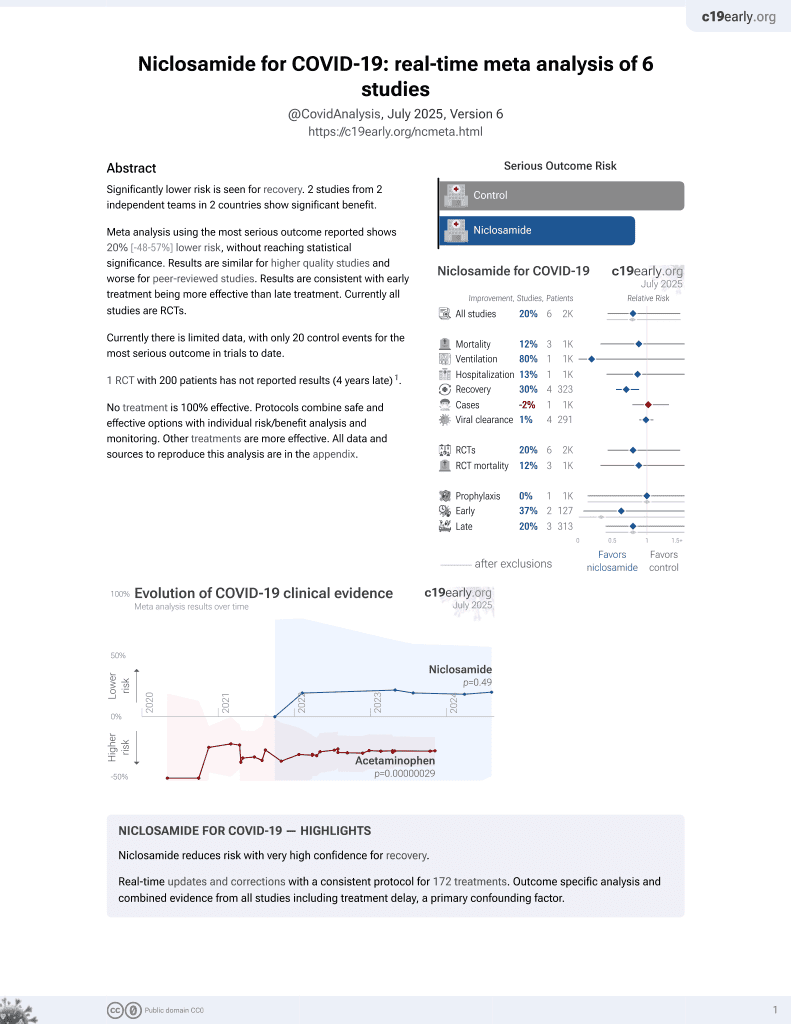
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 46 moderate to severe hospitalized COVID-19 patients showing shorter time to discharge and WHO clinical scale improvement with niclosamide, but no significant difference for resolution of all symptoms and viral clearance.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
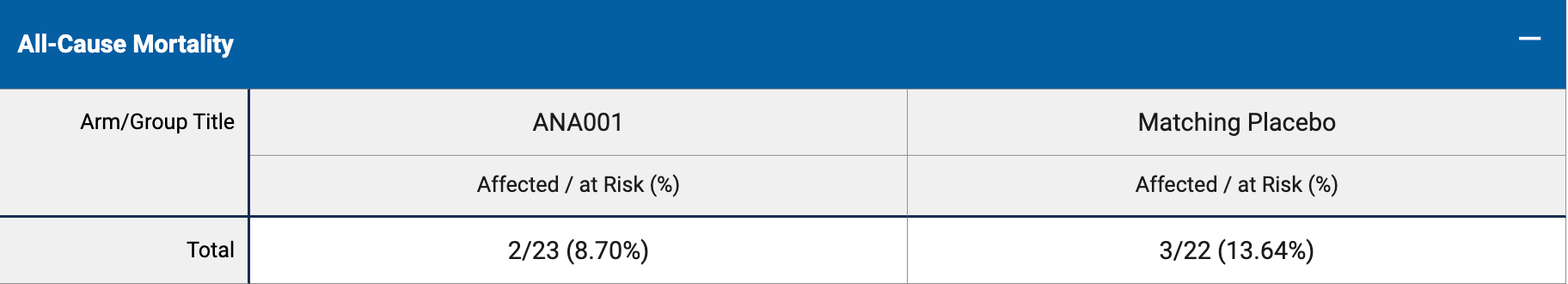
risk of death, 30.3% lower, RR 0.70, p = 1.00, treatment 2 of 22 (9.1%), control 3 of 23 (13.0%), NNT 25.
|
|
recovery time, 70.2% lower, relative time 0.30, p = 0.97, treatment mean 4.15 (±4.87) n=23, control mean 13.91 (±1220) n=23, time to 2 point improvement.
|
|
recovery time, 6.2% higher, relative time 1.06, p = 0.90, treatment mean 17.0 (±33.0) n=23, control mean 16.0 (±15.9) n=23, time to resolution of symptoms.
|
|
time to discharge, 56.7% lower, relative time 0.43, p = 0.98, treatment mean 6.03 (±14.5) n=23, control mean 13.92 (±1220) n=23.
|
|
time to viral-, 0.1% lower, relative time 1.00, p = 1.00, treatment mean 13.09 (±29.6) n=23, control mean 13.1 (±14.8) n=23.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rank et al., 6 Aug 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04603924 (history).