A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19
et al., Nature Communications, doi:10.1038/s41467-025-62423-4, KCT0007307, Aug 2025
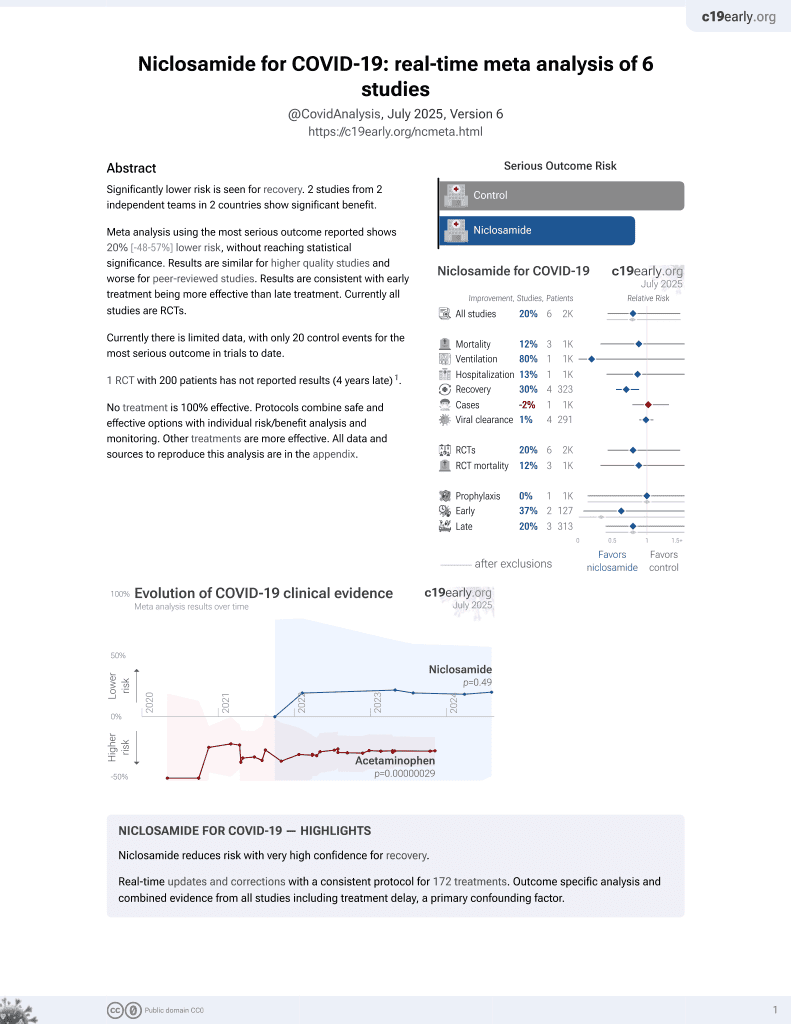
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
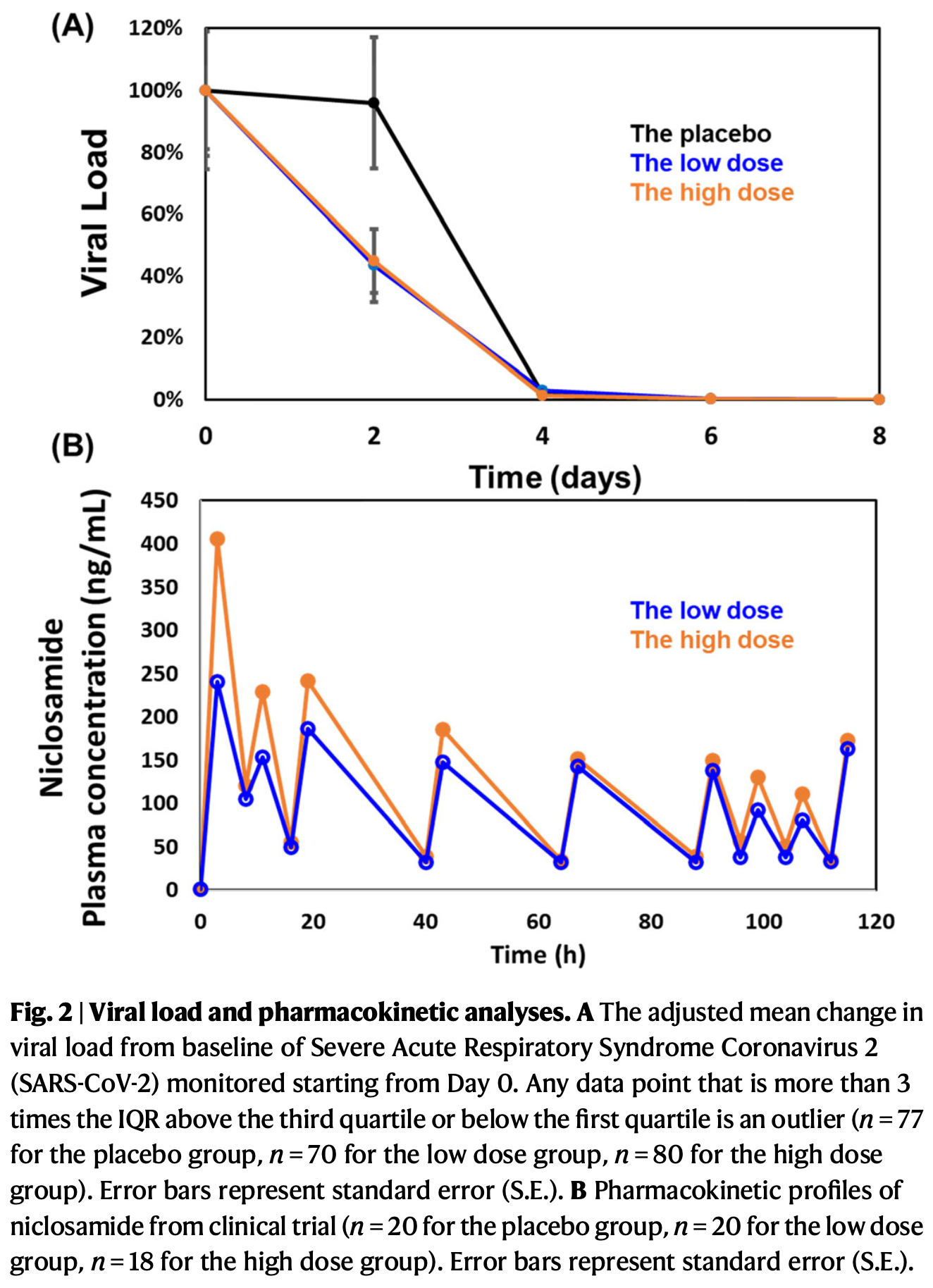
RCT 300 patients with mild to moderate COVID-19 showing significant symptom improvement with niclosamide nanohybrid (CP-COV03). The high-dose group showed no significant benefit in time to symptom improvement in the primary analysis, which authors attribute to gastrointestinal side effects from excess magnesium oxide content confounding symptom assessment. A post-hoc analysis with three COVID-19 representative symptoms showed significant improvement in both groups.
|
recovery time, 29.1% lower, relative time 0.71, p = 0.008, treatment 99, control 98, both groups.
|
|
recovery time, 33.3% lower, relative time 0.67, p = 0.03, treatment mean 4.0 (±2.54) n=99, control mean 6.0 (±8.84) n=98, low dose, post-hoc COVID-19 symptoms.
|
|
recovery time, 25.0% lower, relative time 0.75, p = 0.11, treatment mean 4.5 (±2.5) n=96, control mean 6.0 (±8.84) n=98, high dose, post-hoc COVID-19 symptoms.
|
|
recovery time, 4.1% lower, relative time 0.96, p = 0.80, treatment 99, control 98, both groups.
|
|
recovery time, 22.2% lower, relative time 0.78, p = 0.28, treatment mean 10.5 (±22.8) n=99, control mean 13.5 (±15.2) n=98, low dose, all symptoms, Table S51.
|
|
recovery time, 7.4% higher, relative time 1.07, p = 0.65, treatment mean 14.5 (±15.0) n=96, control mean 13.5 (±15.2) n=98, high dose, all symptoms, Table S51.
|
|
viral load, 55.8% lower, relative load 0.44, p = 0.16, treatment 99, control 98, both groups.
|
|
viral load, 26.4% lower, relative load 0.74, p = 0.77, treatment mean 62918 (±399629) n=99, control mean 46282 (±413674) n=98, low dose, relative reduction in viral load, mid-recovery, day 2.
|
|
viral load, 65.7% lower, relative load 0.34, p = 0.13, treatment mean 135111 (±407691) n=96, control mean 46282 (±413674) n=98, high dose, relative reduction in viral load, mid-recovery, day 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kim et al., 1 Aug 2025, Double Blind Randomized Controlled Trial, placebo-controlled, South Korea, peer-reviewed, mean age 42.6, 12 authors, study period 11 May, 2022 - 28 November, 2022, trial KCT0007307.
Contact: jhchoy@dankook.ac.kr, seran@yuhs.ac.
A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19
Nature Communications, doi:10.1038/s41467-025-62423-4
Effective and reliable treatments for SARS-CoV-2 infections are a key part of global COVID-19 management. Based on vitro studies, niclosamide has been considered as a potential drug candidate for SARS-CoV-2, but its clinical development has been limited due to poor solubility and bioavailability. Here we report results from a randomized, double-blind, placebo-controlled clinical trial involving 300 patients (Clinical Trial Registration Number: KCT0007307) that assessed the efficacy and safety of the niclosamide nanohybrid CP-COV03 at two different doses. Oral CP-COV03 was well tolerated, with no serious adverse events reported in any treatment group. The primary endpoints demonstrated that CP-COV03 significantly alleviated all 12 FDA-recommended COVID-19 symptoms, with symptom improvement sustained for more than 48 h. Additionally, CP-COV03 reduced SARS-CoV-2 viral load by 56.7% within 16 h of the initial dose compared to baseline. Secondary endpoints, including time to sustained symptom resolution, time to return to usual health, and reduction in hospitalization risk, also showed favorable results in the CP-COV03 group compared to placebo. These findings indicate that CP-COV03 is a safe and effective therapeutic option for the treatment of mild to moderate COVID-19 and represents a promising advancement in the repurposing of niclosamide through nanohybrid engineering.
Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability The datasets generated and analyzed during the current study are not publicly available due to clinical data privacy restrictions and ethical considerations involving patient confidentiality. Access to these clinical data may be granted for non-commercial academic research purposes upon reasonable request and is subjected to approval by the study sponsor, Hyundai Bioscience. Requests should be directed to the corresponding authors, Prof. Jin-Ho Choy (e-mail: jhchoy@dankook.ac.kr) and Prof. Jun Yong Choi (e-mail: seran@yuhs.ac). Requests will be evaluated within two weeks, and the data will be available for one month after approval. The source code used for the data analysis and modeling in this study is publicly available at GitHub: https://github. com/jhchoy1/CPCOV03-CODE.git . All other data supporting the findings of this study, including processed results, are available in the Supplementary Information.
Author contributions J.H.K., S.K., S.W.K., D.W.P., K.T.W., J.W.S., were involved in the clinical trial analyses supervised by J.Y.C. along with G.W.J. and J.H.C. The material parts involved synthesis, characterization and analyses were done by S.Y., G.C. and N.S.R. supervised by J.H.C. All authors contributed to the interpretation of the results and have given approval to the final version of the manuscript.
..
References
Andersen, Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine, Viruses, doi:10.3390/v11100964
Butler, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an openlabel, platform-adaptive randomised controlled trial, Lancet
Cairns, Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A Phase 2 Randomized Clinical Trial, JAMA Netw. Open
Carfì, Bernabei, Landi, Group, Persistent symptoms in patients after acute COVID-19, JAMA
Choi, Hydrotalcite-niclosamide nanohybrid as oral formulation towards SARS-CoV-2 viral infections, Pharmaceuticals
Choi, The next generation COVID-19 antiviral; niclosamidebased inorganic nanohybrid system kills SARS-CoV-2, Small, doi:10.1002/smll.202305148
Clark, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study, Lancet Glob. Health
Davis, Long COVID: major findings, mechanisms and recommendations, Nat. Rev. Microbiol
Gallant, Mercier, Rioux-Perreault, Lemaire-Paquette, Piché, Prevalence of persistent symptoms at least 1 month after SARS-CoV-2 omicron infection in adults, J. Assoc. Med. Microbiol. Infect. Dis. Can
Gao, Recent advances in materials for extended-release antibiotic delivery system, J. Antibiot
Gassen, SKP2 attenuates autophagy through Beclin1ubiquitination and its inhibition reduces MERS-coronavirus infection, Nat. Commun
Ghafari, Prevalence of persistent SARS-CoV-2 in a large community surveillance study, Nature
Hammond, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N. Engl. J. Med
Hammond, Sustained Alleviation and Resolution of Targeted COVID-19 Symptoms with Nirmatrelvir/Ritonavir Versus placebo
Harrison, COVID-19 antiviral pills raise hopes for curbing pandemic, Nat. Biotechnol
Harrison, Drug researchers pursue new lines of attack against COVID-19, Nat. Biotechnol
Herbert, Relationship between acute SARS-CoV-2 viral clearance with Long COVID Symptoms: a cohort study, medRxiv, doi:10.1101/2024.07.04.24309953
Huang, Yang, Yuan, Li, Kuang, Corrigendum to Niclosamide inhibits lytic replication of Epstein-Barr virus by disrupting mTOR activation, Antivir. Res
Jacobs, Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters, Clin. Microbiol. Infect
Jalali, Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants, Nat. Commun
Jeon, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob. Agents Chemother
Jurgeit, Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLOS Pathog
Kubota, Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a doubleblind and randomized clinical trial, Nutrients
Marrugal-Lorenzo, Serna-Gallego, Berastegui-Cabrera, Pachón, Sánchez-Céspedes, Repositioning salicylanilide anthelmintic drugs to treat adenovirus infections, Sci. Rep
Mazzon, Identification of broad-spectrum antiviral compounds by targeting viral entry, Viruses, doi:10.3390/v11020176
Menni, Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study, Lancet
Mukae, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob. Agents Chemother
Mukae, Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: The phase 2B part of a randomized, placebo-controlled, phase 2/3 study, Clin. Infect. Dis
Niyomdecha, Suptawiwat, Boonarkart, Thitithanyanont, Auewarakul, Repurposing of antiparasitic niclosamide to inhibit respiratory syncytial virus (RSV) replication, Virus Res, doi:10.1016/j.virusres.2020.198277
Piao, Niclosamide encapsulated in mesoporous silica and geopolymer: A potential oral formulation for COVID-19, Micropor. Mesopor. Mater
Rejinold, Injectable niclosamide nanohybrid as an anti-SARS-CoV-2 strategy, Colloids Surf. B Biointerfaces, doi:10.1016/j.colsurfb.2023.113386
Rejinold, Jin, Choy, Insight into preventing global dengue spread: nanoengineered niclosamide for viral infections, Nano Lett
Saravolatz, Depcinski, Sharma, Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs, Clin. Infect. Dis
Seeßle, Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): A prospective cohort study, Clin. Infect. Dis
Seo, Jin, Yoo, Pharmacokinetic considerations for enhancing drug repurposing opportunities of anthelmintics: Niclosamide as a case study, Biomed. Pharmacother
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: A systematic review of literature, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2021.102329
Standing, Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nat. Commun, doi:10.1038/s41467-024-45641-0
Tam, Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota, Nat. Commun
Tao, Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice, Nat. Med
Udy, Clinical implications of antibiotic pharmacokinetic principles in the critically ill, Intensive Care Med
Wang, Early administration of Paxlovid® reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants, J. Med. Virol, doi:10.1002/jmv.28443
Wanounou, Caraco, Levy, Bialer, Perucca, Clinically relevant interactions between ritonavir-boosted nirmatrelvir and concomitant antiseizure medications: implications for the management of COVID-19 in patients with epilepsy, Clin. Pharmacokinet
Wei, Risk of SARS-CoV-2 reinfection during multiple Omicron variant waves in the UK general population, Nat. Commun, doi:10.1038/s41467-024-44973-1
Weiss, Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B. 1.1. 7), Beta (B. 1.351) and Delta variant (B. 1.617. 2), PloS one
Wu, Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob. Agents Chemother
Xu, Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen, Nat. Med
Yang, Association of SARS-CoV-2 infection and persistence with long COVID, Lancet Respir. Med
Yu, Niclosamide-clay intercalate coated with nonionic polymer for enhanced bioavailability toward COVID-19 treatment, Polymers
DOI record:
{
"DOI": "10.1038/s41467-025-62423-4",
"ISSN": [
"2041-1723"
],
"URL": "http://dx.doi.org/10.1038/s41467-025-62423-4",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Effective and reliable treatments for SARS-CoV-2 infections are a key part of global COVID-19 management. Based on vitro studies, niclosamide has been considered as a potential drug candidate for SARS-CoV-2, but its clinical development has been limited due to poor solubility and bioavailability. Here we report results from a randomized, double-blind, placebo-controlled clinical trial involving 300 patients (Clinical Trial Registration Number: KCT0007307) that assessed the efficacy and safety of the niclosamide nanohybrid CP-COV03 at two different doses. Oral CP-COV03 was well tolerated, with no serious adverse events reported in any treatment group. The primary endpoints demonstrated that CP-COV03 significantly alleviated all 12 FDA-recommended COVID-19 symptoms, with symptom improvement sustained for more than 48 h. Additionally, CP-COV03 reduced SARS-CoV-2 viral load by 56.7% within 16 h of the initial dose compared to baseline. Secondary endpoints, including time to sustained symptom resolution, time to return to usual health, and reduction in hospitalization risk, also showed favorable results in the CP-COV03 group compared to placebo. These findings indicate that CP-COV03 is a safe and effective therapeutic option for the treatment of mild to moderate COVID-19 and represents a promising advancement in the repurposing of niclosamide through nanohybrid engineering.</jats:p>",
"alternative-id": [
"62423"
],
"article-number": "7084",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 June 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "22 July 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 August 2025"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-5033-3482",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jung Ho",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kym",
"given": "Sungmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Shin-Woo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Dae Won",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kwon",
"given": "Ki Tae",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2806-1863",
"affiliation": [],
"authenticated-orcid": false,
"family": "Seo",
"given": "Jun-Won",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Seungjin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Goeun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "N",
"given": "Sanoj Rejinold",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4149-7100",
"affiliation": [],
"authenticated-orcid": false,
"family": "Choy",
"given": "Jin-Ho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jin",
"given": "Geun-woo",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2775-3315",
"affiliation": [],
"authenticated-orcid": false,
"family": "Choi",
"given": "Jun Yong",
"sequence": "additional"
}
],
"container-title": "Nature Communications",
"container-title-short": "Nat Commun",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
8,
1
]
],
"date-time": "2025-08-01T18:15:25Z",
"timestamp": 1754072125000
},
"deposited": {
"date-parts": [
[
2025,
8,
1
]
],
"date-time": "2025-08-01T18:15:31Z",
"timestamp": 1754072131000
},
"indexed": {
"date-parts": [
[
2025,
8,
2
]
],
"date-time": "2025-08-02T00:11:51Z",
"timestamp": 1754093511461,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
8,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
1
]
],
"date-time": "2025-08-01T00:00:00Z",
"timestamp": 1754006400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
1
]
],
"date-time": "2025-08-01T00:00:00Z",
"timestamp": 1754006400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41467-025-62423-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41467-025-62423-4",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41467-025-62423-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
8,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S2214-109X(20)30264-3",
"author": "A Clark",
"doi-asserted-by": "crossref",
"first-page": "e1003",
"journal-title": "Lancet Glob. Health",
"key": "62423_CR1",
"unstructured": "Clark, A. et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob. Health 8, e1003–e1017 (2020).",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41467-022-33233-9",
"author": "N Jalali",
"doi-asserted-by": "crossref",
"journal-title": "Nat. Commun.",
"key": "62423_CR2",
"unstructured": "Jalali, N. et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat. Commun. 13, 5706 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41467-024-44973-1",
"doi-asserted-by": "publisher",
"key": "62423_CR3",
"unstructured": "Wei, J. et al. Risk of SARS-CoV-2 reinfection during multiple Omicron variant waves in the UK general population. Nat. Commun. 15 https://doi.org/10.1038/s41467-024-44973-1 (2024)."
},
{
"DOI": "10.1038/d41587-020-00013-z",
"author": "C Harrison",
"doi-asserted-by": "crossref",
"first-page": "659",
"journal-title": "Nat. Biotechnol.",
"key": "62423_CR4",
"unstructured": "Harrison, C. Drug researchers pursue new lines of attack against COVID-19. Nat. Biotechnol. 38, 659–662 (2020).",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1038/d41587-020-00013-z",
"author": "C Harrison",
"doi-asserted-by": "crossref",
"first-page": "659",
"journal-title": "Nat. Biotechnol.",
"key": "62423_CR5",
"unstructured": "Harrison, C. COVID-19 antiviral pills raise hopes for curbing pandemic. Nat. Biotechnol. 38, 659–662 (2021).",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2021.102329",
"doi-asserted-by": "publisher",
"key": "62423_CR6",
"unstructured": "Singh, A. K., Singh, A., Singh, R. & Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. Clin. Res. Rev. 15, https://doi.org/10.1016/j.dsx.2021.102329 (2021)."
},
{
"DOI": "10.1093/cid/ciac180",
"author": "LD Saravolatz",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Clin. Infect. Dis.",
"key": "62423_CR7",
"unstructured": "Saravolatz, L. D., Depcinski, S. & Sharma, M. Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin. Infect. Dis. 76, 165–171 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac933",
"author": "H Mukae",
"doi-asserted-by": "crossref",
"first-page": "1403",
"journal-title": "Clin. Infect. Dis.",
"key": "62423_CR8",
"unstructured": "Mukae, H. et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: The phase 2B part of a randomized, placebo-controlled, phase 2/3 study. Clin. Infect. Dis. 76, 1403–1411 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1007/s40262-022-01152-z",
"author": "M Wanounou",
"doi-asserted-by": "crossref",
"first-page": "1219",
"journal-title": "Clin. Pharmacokinet.",
"key": "62423_CR9",
"unstructured": "Wanounou, M., Caraco, Y., Levy, R. H., Bialer, M. & Perucca, E. Clinically relevant interactions between ritonavir-boosted nirmatrelvir and concomitant antiseizure medications: implications for the management of COVID-19 in patients with epilepsy. Clin. Pharmacokinet. 61, 1219–1236 (2022).",
"volume": "61",
"year": "2022"
},
{
"DOI": "10.1038/s41467-024-45641-0",
"doi-asserted-by": "publisher",
"key": "62423_CR10",
"unstructured": "Standing, J. F. et al. Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients. Nat. Commun. 15, https://doi.org/10.1038/s41467-024-45641-0 (2024)."
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "62423_CR11",
"unstructured": "Butler, C. C. et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 401, 281–293 (2023).",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1128/AAC.00819-20",
"author": "S Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "62423_CR12",
"unstructured": "Jeon, S. et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 64, e00819–00820 (2020).",
"volume": "64",
"year": "2020"
},
{
"author": "Anne Weiss",
"journal-title": "PloS one",
"key": "62423_CR13",
"unstructured": "Weiss, A. nne. et al. Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B. 1.1. 7), Beta (B. 1.351) and Delta variant (B. 1.617. 2). PloS one. 16, e0260958 (2021).",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41467-019-13659-4",
"author": "NC Gassen",
"doi-asserted-by": "crossref",
"journal-title": "Nat. Commun.",
"key": "62423_CR14",
"unstructured": "Gassen, N. C. et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat. Commun. 10, 5770 (2019).",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1128/AAC.48.7.2693-2696.2004",
"author": "CJ Wu",
"doi-asserted-by": "crossref",
"first-page": "2693",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "62423_CR15",
"unstructured": "Wu, C. J. et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 48, 2693–2696 (2004).",
"volume": "48",
"year": "2004"
},
{
"DOI": "10.1038/s41598-018-37290-3",
"author": "JA Marrugal-Lorenzo",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "62423_CR16",
"unstructured": "Marrugal-Lorenzo, J. A., Serna-Gallego, A., Berastegui-Cabrera, J., Pachón, J. & Sánchez-Céspedes, J. Repositioning salicylanilide anthelmintic drugs to treat adenovirus infections. Sci. Rep. 9, 17 (2019).",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.3390/v11020176",
"doi-asserted-by": "publisher",
"key": "62423_CR17",
"unstructured": "Mazzon, M. et al. Identification of broad-spectrum antiviral compounds by targeting viral entry. Viruses 11, https://doi.org/10.3390/v11020176 (2019)."
},
{
"DOI": "10.1371/journal.ppat.1002976",
"author": "A Jurgeit",
"doi-asserted-by": "crossref",
"first-page": "e1002976",
"journal-title": "PLOS Pathog.",
"key": "62423_CR18",
"unstructured": "Jurgeit, A. et al. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLOS Pathog. 8, e1002976 (2012).",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.3390/v11100964",
"doi-asserted-by": "publisher",
"key": "62423_CR19",
"unstructured": "Andersen, P. I. et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses 11, https://doi.org/10.3390/v11100964 (2019)."
},
{
"DOI": "10.1016/j.antiviral.2017.01.019",
"author": "L Huang",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "Antivir. Res",
"key": "62423_CR20",
"unstructured": "Huang, L., Yang, M. T., Yuan, Y., Li, X. J. & Kuang, E. S. Corrigendum to Niclosamide inhibits lytic replication of Epstein-Barr virus by disrupting mTOR activation. Antivir. Res. 140, 164–165 (2017).",
"volume": "140",
"year": "2017"
},
{
"DOI": "10.1016/j.virusres.2020.198277",
"doi-asserted-by": "publisher",
"key": "62423_CR21",
"unstructured": "Niyomdecha, N., Suptawiwat, O., Boonarkart, C., Thitithanyanont, A. & Auewarakul, P. Repurposing of antiparasitic niclosamide to inhibit respiratory syncytial virus (RSV) replication. Virus Res. 295, https://doi.org/10.1016/j.virusres.2020.198277 (2021)."
},
{
"DOI": "10.1038/nm.4184",
"author": "M Xu",
"doi-asserted-by": "crossref",
"first-page": "1101",
"journal-title": "Nat. Med.",
"key": "62423_CR22",
"unstructured": "Xu, M. et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 22, 1101–1107 (2016).",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1038/nm.3699",
"author": "H Tao",
"doi-asserted-by": "crossref",
"first-page": "1263",
"journal-title": "Nat. Med.",
"key": "62423_CR23",
"unstructured": "Tao, H. et al. Niclosamide ethanolamine–induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20, 1263–1269 (2014).",
"volume": "20",
"year": "2014"
},
{
"author": "John Tam",
"journal-title": "Nat. Commun.",
"key": "62423_CR24",
"unstructured": "Tam, J. et al. Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nat. Commun. 9, 5233 (2018).",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1021/acs.nanolett.4c02845",
"doi-asserted-by": "crossref",
"key": "62423_CR25",
"unstructured": "Rejinold, N. S., Jin, G.-w. & Choy, J.-H. Insight into preventing global dengue spread: nanoengineered niclosamide for viral infections. Nano Lett. (2024) in press."
},
{
"DOI": "10.1016/j.biopha.2024.116394",
"author": "JI Seo",
"doi-asserted-by": "crossref",
"first-page": "116394",
"journal-title": "Biomed. Pharmacother.",
"key": "62423_CR26",
"unstructured": "Seo, J. I., Jin, G. -w & Yoo, H. H. Pharmacokinetic considerations for enhancing drug repurposing opportunities of anthelmintics: Niclosamide as a case study. Biomed. Pharmacother. 173, 116394 (2024).",
"volume": "173",
"year": "2024"
},
{
"DOI": "10.3390/ph14050486",
"author": "G Choi",
"doi-asserted-by": "crossref",
"first-page": "486",
"journal-title": "Pharmaceuticals",
"key": "62423_CR27",
"unstructured": "Choi, G. et al. Hydrotalcite–niclosamide nanohybrid as oral formulation towards SARS-CoV-2 viral infections. Pharmaceuticals 14, 486 (2021).",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.3390/polym13071044",
"author": "S Yu",
"doi-asserted-by": "crossref",
"first-page": "1044",
"journal-title": "Polymers",
"key": "62423_CR28",
"unstructured": "Yu, S. et al. Niclosamide–clay intercalate coated with nonionic polymer for enhanced bioavailability toward COVID-19 treatment. Polymers 13, 1044 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.colsurfb.2023.113386",
"doi-asserted-by": "publisher",
"key": "62423_CR29",
"unstructured": "Rejinold, N. S. et al. Injectable niclosamide nanohybrid as an anti-SARS-CoV-2 strategy. Colloids Surf. B Biointerfaces 112063 https://doi.org/10.1016/j.colsurfb.2023.113386 (2021)."
},
{
"DOI": "10.1016/j.micromeso.2021.111394",
"author": "H Piao",
"doi-asserted-by": "crossref",
"first-page": "111394",
"journal-title": "Micropor. Mesopor. Mater.",
"key": "62423_CR30",
"unstructured": "Piao, H. et al. Niclosamide encapsulated in mesoporous silica and geopolymer: A potential oral formulation for COVID-19. Micropor. Mesopor. Mater. 326, 111394 (2021).",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"author": "DM Cairns",
"doi-asserted-by": "crossref",
"first-page": "e2144942",
"journal-title": "JAMA Netw. Open",
"key": "62423_CR31",
"unstructured": "Cairns, D. M. et al. Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 5, e2144942 (2022).",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1002/smll.202305148",
"doi-asserted-by": "publisher",
"key": "62423_CR32",
"unstructured": "Choi, G. et al. The next generation COVID-19 antiviral; niclosamide-based inorganic nanohybrid system kills SARS-CoV-2. Small, e2305148 https://doi.org/10.1002/smll.202305148 (2023)."
},
{
"DOI": "10.1016/S0140-6736(22)00327-0",
"author": "C Menni",
"doi-asserted-by": "crossref",
"first-page": "1618",
"journal-title": "Lancet",
"key": "62423_CR33",
"unstructured": "Menni, C. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 399, 1618–1624 (2022).",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1007/s00134-013-3088-4",
"author": "AA Udy",
"doi-asserted-by": "crossref",
"first-page": "2070",
"journal-title": "Intensive Care Med",
"key": "62423_CR34",
"unstructured": "Udy, A. A. et al. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 39, 2070–2082 (2013).",
"volume": "39",
"year": "2013"
},
{
"DOI": "10.1038/ja.2011.58",
"author": "P Gao",
"doi-asserted-by": "crossref",
"first-page": "625",
"journal-title": "J. Antibiot.",
"key": "62423_CR35",
"unstructured": "Gao, P. et al. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 64, 625–634 (2011).",
"volume": "64",
"year": "2011"
},
{
"DOI": "10.1046/j.1198-743x.2001.00295.x",
"author": "MR Jacobs",
"doi-asserted-by": "crossref",
"first-page": "589",
"journal-title": "Clin. Microbiol. Infect.",
"key": "62423_CR36",
"unstructured": "Jacobs, M. R. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7, 589–596 (2001).",
"volume": "7",
"year": "2001"
},
{
"DOI": "10.1002/jmv.28443",
"doi-asserted-by": "publisher",
"key": "62423_CR37",
"unstructured": "Wang, Y. et al. Early administration of Paxlovid® reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants. J. Med. Virol. 95, https://doi.org/10.1002/jmv.28443 (2023)."
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "62423_CR38",
"unstructured": "Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1128/aac.00697-22",
"author": "H Mukae",
"doi-asserted-by": "crossref",
"first-page": "e00697",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "62423_CR39",
"unstructured": "Mukae, H. et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob. Agents Chemother. 66, e00697–00622 (2022).",
"volume": "66",
"year": "2022"
},
{
"key": "62423_CR40",
"unstructured": "Omeprazole Dosage Guide + max dose, adjustments (no date) Drugs.com. Available at: https://www.drugs.com/dosage/omeprazole.html#Usual_Adult_Dose_for_Dyspepsia (Accessed: 13 September (2024)."
},
{
"DOI": "10.3390/nu12010225",
"author": "M Kubota",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Nutrients",
"key": "62423_CR41",
"unstructured": "Kubota, M. et al. Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a double-blind and randomized clinical trial. Nutrients 12, 225 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1101/2024.07.04.24309953",
"doi-asserted-by": "publisher",
"key": "62423_CR42",
"unstructured": "Herbert, C. B. et al. Relationship between acute SARS-CoV-2 viral clearance with Long COVID Symptoms: a cohort study. medRxiv 2024-07 https://doi.org/10.1101/2024.07.04.24309953 (2024)."
},
{
"DOI": "10.1016/S2213-2600(23)00142-X",
"author": "Chengliang Yang",
"doi-asserted-by": "crossref",
"first-page": "504",
"journal-title": "Lancet Respir. Med.",
"key": "62423_CR43",
"unstructured": "Yang, C. hengliang. et al. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir. Med. 11, 504–506 (2023).",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1038/s41586-024-07029-4",
"author": "M Ghafari",
"doi-asserted-by": "crossref",
"first-page": "1094",
"journal-title": "Nature",
"key": "62423_CR44",
"unstructured": "Ghafari, M. et al. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 626, 1094–1101 (2024).",
"volume": "626",
"year": "2024"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"author": "HE Davis",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Nat. Rev. Microbiol.",
"key": "62423_CR45",
"unstructured": "Davis, H. E. et al. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciab611",
"author": "J Seeßle",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin. Infect. Dis.",
"key": "62423_CR46",
"unstructured": "Seeßle, J. et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): A prospective cohort study. Clin. Infect. Dis. 74, 1191–1198 (2022).",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.12603",
"author": "A Carfì",
"doi-asserted-by": "crossref",
"first-page": "603",
"journal-title": "JAMA",
"key": "62423_CR47",
"unstructured": "Carfì, A., Bernabei, R. & Landi, F. & Group, f.t.G.A.C.-P.-A.C.S. Persistent symptoms in patients after acute COVID-19. JAMA. 324, 603–605 (2020).",
"volume": "324",
"year": "2020"
},
{
"author": "M Gallant",
"first-page": "57",
"journal-title": "J. Assoc. Med. Microbiol. Infect. Dis. Can.",
"key": "62423_CR48",
"unstructured": "Gallant, M., Mercier, K., Rioux-Perreault, C., Lemaire-Paquette, S. & Piché, A. Prevalence of persistent symptoms at least 1 month after SARS-CoV-2 omicron infection in adults. J. Assoc. Med. Microbiol. Infect. Dis. Can. 8, 57–63 (2023).",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac492.994",
"doi-asserted-by": "crossref",
"key": "62423_CR49",
"unstructured": "Hammond, J. et al Sustained Alleviation and Resolution of Targeted COVID-19 Symptoms with Nirmatrelvir/Ritonavir Versus placebo. Poster presented at IDWeek 2022, 19-23 October 2022, Washington, DC, USA."
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41467-025-62423-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A randomized, double-blind, placebo-controlled trial of niclosamide nanohybrid for the treatment of patients with mild to moderate COVID-19",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "16"
}