Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.102359, NCT05620160, Jan 2024
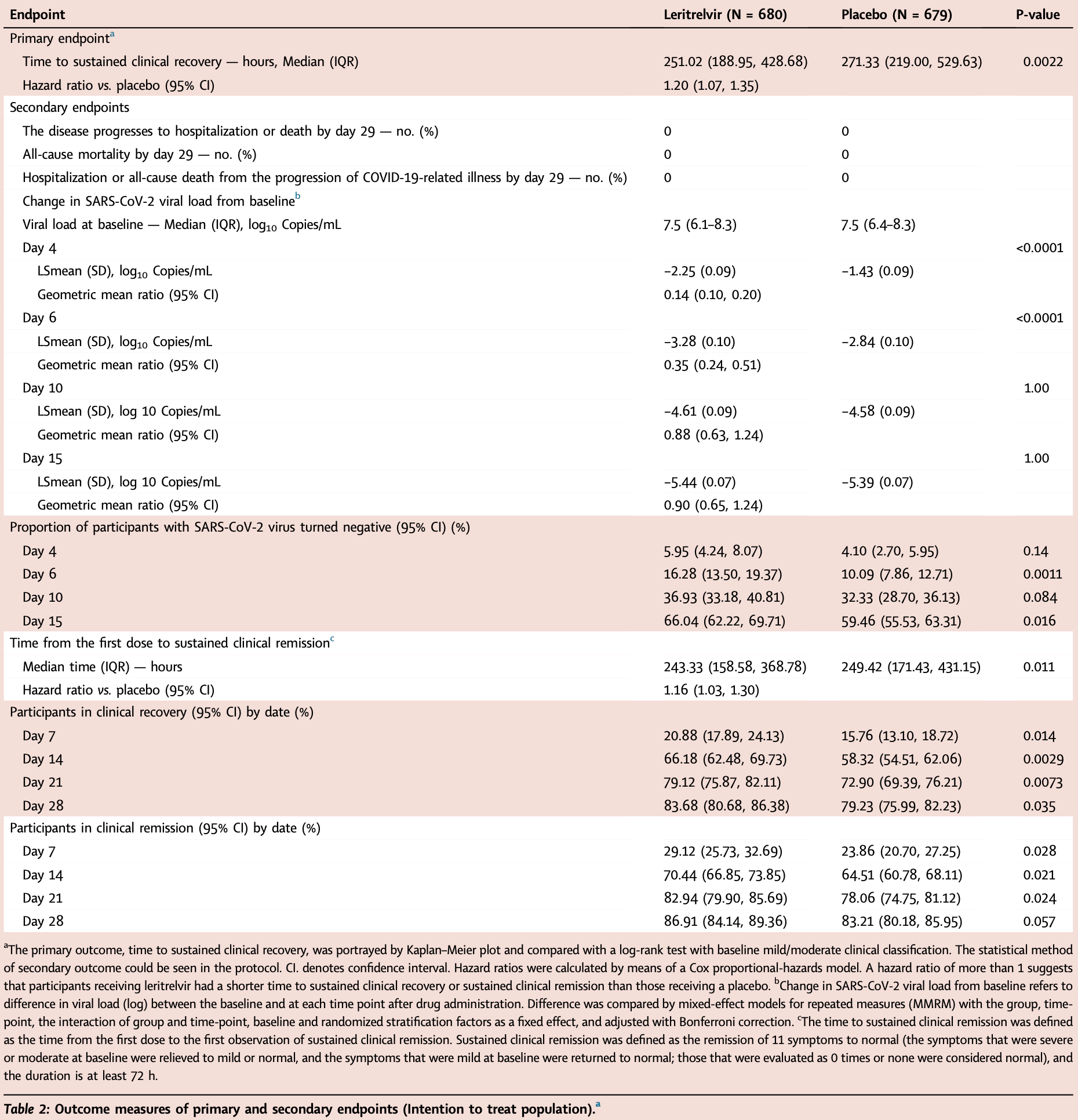
RCT 1,359 COVID-19 outpatients showing faster recovery with leritrelvir monotherapy (without ritonavir), 251 vs. 271 hours, and improved viral clearance. There were no significant differences in adverse events between groups.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
sustained clinical recovery, 16.7% lower, HR 0.83, p = 0.002, treatment 680, control 679, inverted to make HR<1 favor treatment.
|
|
sustained clinical remission, 13.8% lower, HR 0.86, p = 0.01, treatment 680, control 679, inverted to make HR<1 favor treatment.
|
|
clinical recovery, 21.4% lower, RR 0.79, p = 0.04, treatment 111 of 680 (16.3%), control 141 of 679 (20.8%), NNT 23, day 28.
|
|
clinical remission, 22.0% lower, RR 0.78, p = 0.06, treatment 89 of 680 (13.1%), control 114 of 679 (16.8%), NNT 27, day 28.
|
|
risk of no viral clearance, 16.1% lower, RR 0.84, p = 0.01, treatment 231 of 680 (34.0%), control 275 of 679 (40.5%), NNT 15, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhan et al., 31 Jan 2024, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, 45 authors, study period 12 November, 2022 - 30 December, 2022, trial NCT05620160 (history).
Contact: jeffyah@163.com, nanshan@vip.163.com, jpzhenggy@163.com.
Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial
eClinicalMedicine, doi:10.1016/j.eclinm.2023.102359
Background Leritrelvir is a novel α-ketoamide based peptidomimetic inhibitor of SARS-CoV-2 main protease. A preclinical study has demonstrated leritrelvir poses similar antiviral activities towards different SARS-CoV-2 variants compared with nirmatrelvir. A phase 2 clinical trial has shown a comparable antiviral efficacy and safety between leritrelvir with and without ritonavir co-administration. This trial aims to test efficacy and safety of leritrelvir monotherapy in adults with mild-to-moderate COVID-19.
Contributors Conceived study: Nanshan Zhong, Jingping Zheng, Zifeng Yang, Designed study and experiments: Yangqing Zhan, Zifeng Yang, Nanshan Zhong. Performed experiments: Yangqing Zhan, Nanshan Zhong, Jingping Zheng, Yueping Li, Hongzhou Lu, Ling Lin, Liang Su, Tianxin Xiang, Hongqiu Pan, Chaolin Huang, Ying Deng, Furong Wang, Ruhong Xu, Bingliang Lin, Ruilin Sun, Fangqi Ge, Dexiong Chen, Ping Zhang, Jianlin Tong, Xifu Wang, Qingwei Meng, Zhigang Zheng, Shuqiang Ou, Xiaoyun Guo, Herui Yao, Tao Yu, Weiyang Li, Yu Zhang. Interpreted data: Zhengshi Lin, Nanshan Zhong, Yangqing Zhan, Jingyi Liang, Zhonghao Fang, Shiwei Liang, Haijun Li. Manuscript preparation: Zhengshi Lin, Chuanmeizi Tu, Qianying Li, Zifeng Yang, Nanshan Zhong, Ruifeng Chen, Jingyi Liang, Zhonghao Fang, Jincan Luo, Yudi Song, Changyuan Kang, Mei Jiang. Jingyi Liang, Zhonghao Fang, Shiwiei Liang, Zhengshi Lin have accessed and verified the data, and Zifeng Yang, Jingping Zheng, Nanshan Zhong were responsible for the decision to submit the manuscript.
Data sharing statement After approval from Human Genetic Resource Adminstration of China and Guangdong Raynovent Biotech Co., Ltd., this trial data can be shared with qualifying researchers who submit a valuable research question.
Declaration of interests No potential conflicts of interest were reported by the authors.
References
Cao, Jian, Wang, Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution, Nature
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Cao, Yisimayi, Jian, 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature
Chen, Huang, Ma, Inhibition mechanism and antiviral activity of an α-ketoamide based SARS-CoV-2 main protease inhibitor, bioRxiv, doi:10.1101/2023.03.09.531862
Cui, Liu, Wang, Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron, Cell
Davies, Abbott, Barnard, Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England, Science
Focosi, Mcconnell, Shoham, Casadevall, Maggi et al., Nirmatrelvir and COVID-19: development, pharmacokinetics, clinical efficacy, resistance, relapse, and pharmacoeconomics, Int J Antimicrob Agents
Girardin, Manuel, Marzolini, Buclin, Evaluating the risk of drug-drug interactions with pharmacokinetic boosters: the case of ritonavir-enhanced nirmatrelvir to prevent severe COVID-19, Clin Microbiol Infect
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med
Haque, Mahar, Hussain, Sloane, Pharmacokinetic interaction between verapamil and ritonavir-boosted nirmatrelvir: implications for the management of COVID-19 in patients with hypertension, BMJ Case Rep
Heskin, Pallett, Mughal, Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management, Lancet
Hopkins, Sorich, Mclachlan, Understanding the risk of drug interactions between ritonavir-containing COVID-19 therapies and small-molecule kinase inhibitors in patients with cancer, JCO Precis Oncol
Lamb, Nirmatrelvir plus ritonavir: first approval, Drugs
Li, Yu, Drug-induced liver injury with ritonavir-boosted nirmatrelvir: evidence from coronavirus disease 2019 emergency use authorization adverse event reporting system, Gastroenterology
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin Pharmacol Ther
Mukae, Yotsuyanagi, Ohmagari, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob Agents Chemother
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis
Nih, Therapeutic management of nonhospitalized adults with COVID-19
Owen, Allerton, Anderson, An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science
Wang, Guo, Iketani, Antibody evasion by SARS-CoV-2 Omicron subvariants BA, BA.4 and BA.5
Wang, Li, Cai, Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, doubleblind, placebo-controlled trial, eClinicalMedicine, doi:10.1016/j.eclinm.2023.102189
Who, China, WHO coronavirus (COVID-19) dashboard
Xu, Xie, Al-Aly, Risks and burdens of incident dyslipidaemia in long COVID: a cohort study, Lancet Diabetes Endocrinol
Young, Papiro, Greenberg, Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant, Pediatr Nephrol
DOI record:
{
"DOI": "10.1016/j.eclinm.2023.102359",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2023.102359",
"alternative-id": [
"S2589537023005369"
],
"article-number": "102359",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2023.102359"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Zhan",
"given": "Yangqing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lin",
"given": "Zhengshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Jingyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Ruilin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yueping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Bingliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ge",
"given": "Fangqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Ling",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8308-5534",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lu",
"given": "Hongzhou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Su",
"given": "Liang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xiang",
"given": "Tianxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pan",
"given": "Hongqiu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Chaolin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deng",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Furong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Ruhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Dexiong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tong",
"given": "Jianlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xifu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meng",
"given": "Qingwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Zhigang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ou",
"given": "Shuqiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Xiaoyun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yao",
"given": "Herui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Tao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Weiyang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7393-2664",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jiang",
"given": "Mei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fang",
"given": "Zhonghao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Yudi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Ruifeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Jincan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Changyuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Shiwei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0007-7432-8454",
"affiliation": [],
"authenticated-orcid": false,
"family": "Li",
"given": "Haijun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Jingping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Nanshan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2681-4171",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yang",
"given": "Zifeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yanming",
"given": "Huang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haiping",
"given": "Dong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hou",
"given": "Jinlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lei",
"given": "Shao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xiaoguang",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Gao",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T00:02:30Z",
"timestamp": 1702598550000
},
"deposited": {
"date-parts": [
[
2024,
3,
17
]
],
"date-time": "2024-03-17T10:33:50Z",
"timestamp": 1710671630000
},
"indexed": {
"date-parts": [
[
2024,
6,
15
]
],
"date-time": "2024-06-15T17:19:40Z",
"timestamp": 1718471980793
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T00:00:00Z",
"timestamp": 1700524800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537023005369?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537023005369?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "102359",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England",
"author": "Davies",
"first-page": "372",
"issue": "6538",
"journal-title": "Science",
"key": "10.1016/j.eclinm.2023.102359_bib1",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "657",
"issue": "7898",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2023.102359_bib2",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"article-title": "BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "7923",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2023.102359_bib3",
"volume": "608",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2022.01.019",
"article-title": "Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron",
"author": "Cui",
"doi-asserted-by": "crossref",
"first-page": "860",
"issue": "5",
"journal-title": "Cell",
"key": "10.1016/j.eclinm.2023.102359_bib4",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-05053-w",
"article-title": "Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "603",
"issue": "7923",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2023.102359_bib5",
"volume": "608",
"year": "2022"
},
{
"article-title": "Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution",
"author": "Cao",
"first-page": "521",
"issue": "7948",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2023.102359_bib6",
"volume": "614",
"year": "2023"
},
{
"author": "NIH",
"key": "10.1016/j.eclinm.2023.102359_bib7",
"series-title": "Therapeutic management of nonhospitalized adults with COVID-19",
"year": "2022"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"issue": "6575",
"journal-title": "Science",
"key": "10.1016/j.eclinm.2023.102359_bib8",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1007/s40265-022-01692-5",
"article-title": "Nirmatrelvir plus ritonavir: first approval",
"author": "Lamb",
"doi-asserted-by": "crossref",
"first-page": "585",
"issue": "5",
"journal-title": "Drugs",
"key": "10.1016/j.eclinm.2023.102359_bib9",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1007/s00467-022-05712-0",
"article-title": "Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant",
"author": "Young",
"doi-asserted-by": "crossref",
"first-page": "1387",
"issue": "4",
"journal-title": "Pediatr Nephrol",
"key": "10.1016/j.eclinm.2023.102359_bib10",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1053/j.gastro.2023.02.008",
"article-title": "Drug-induced liver injury with ritonavir-boosted nirmatrelvir: evidence from coronavirus disease 2019 emergency use authorization adverse event reporting system",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Gastroenterology",
"key": "10.1016/j.eclinm.2023.102359_bib11",
"volume": "165",
"year": "2023"
},
{
"article-title": "Understanding the risk of drug interactions between ritonavir-containing COVID-19 therapies and small-molecule kinase inhibitors in patients with cancer",
"author": "Hopkins",
"journal-title": "JCO Precis Oncol",
"key": "10.1016/j.eclinm.2023.102359_bib12",
"volume": "7",
"year": "2023"
},
{
"DOI": "10.1136/bcr-2022-252677",
"article-title": "Pharmacokinetic interaction between verapamil and ritonavir-boosted nirmatrelvir: implications for the management of COVID-19 in patients with hypertension",
"author": "Haque",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "BMJ Case Rep",
"key": "10.1016/j.eclinm.2023.102359_bib13",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2022.03.030",
"article-title": "Evaluating the risk of drug-drug interactions with pharmacokinetic boosters: the case of ritonavir-enhanced nirmatrelvir to prevent severe COVID-19",
"author": "Girardin",
"doi-asserted-by": "crossref",
"first-page": "1044",
"issue": "8",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.eclinm.2023.102359_bib14",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)02657-X",
"article-title": "Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management",
"author": "Heskin",
"doi-asserted-by": "crossref",
"first-page": "21",
"issue": "10319",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2023.102359_bib15",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.ijantimicag.2022.106708",
"article-title": "Nirmatrelvir and COVID-19: development, pharmacokinetics, clinical efficacy, resistance, relapse, and pharmacoeconomics",
"author": "Focosi",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.eclinm.2023.102359_bib16",
"volume": "61",
"year": "2023"
},
{
"article-title": "Inhibition mechanism and antiviral activity of an α-ketoamide based SARS-CoV-2 main protease inhibitor",
"author": "Chen",
"journal-title": "bioRxiv",
"key": "10.1016/j.eclinm.2023.102359_bib17",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.102189",
"article-title": "Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, double-blind, placebo-controlled trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/j.eclinm.2023.102359_bib18",
"volume": "63",
"year": "2023"
},
{
"key": "10.1016/j.eclinm.2023.102359_bib19",
"series-title": "China: WHO coronavirus (COVID-19) dashboard",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.102359_bib20",
"volume": "386",
"year": "2022"
},
{
"author": "Fact Sheet For Healthcare Providers",
"key": "10.1016/j.eclinm.2023.102359_bib22",
"series-title": "Emergency use authorization for paxlovid",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study",
"author": "Mukae",
"doi-asserted-by": "crossref",
"first-page": "1403",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eclinm.2023.102359_bib23",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1128/aac.00697-22",
"article-title": "A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part",
"author": "Mukae",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.eclinm.2023.102359_bib24",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1016/S2213-8587(22)00355-2",
"article-title": "Risks and burdens of incident dyslipidaemia in long COVID: a cohort study",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "120",
"issue": "2",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "10.1016/j.eclinm.2023.102359_bib25",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"issue": "6",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.eclinm.2023.102359_bib27",
"volume": "112",
"year": "2022"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537023005369"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "67"
}