Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806, NCT05667714, May 2023
SA58 for COVID-19
55th treatment shown to reduce risk in
December 2023, now with p = 0.018 from 3 studies.
Efficacy is variant dependent.
Lower risk for cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
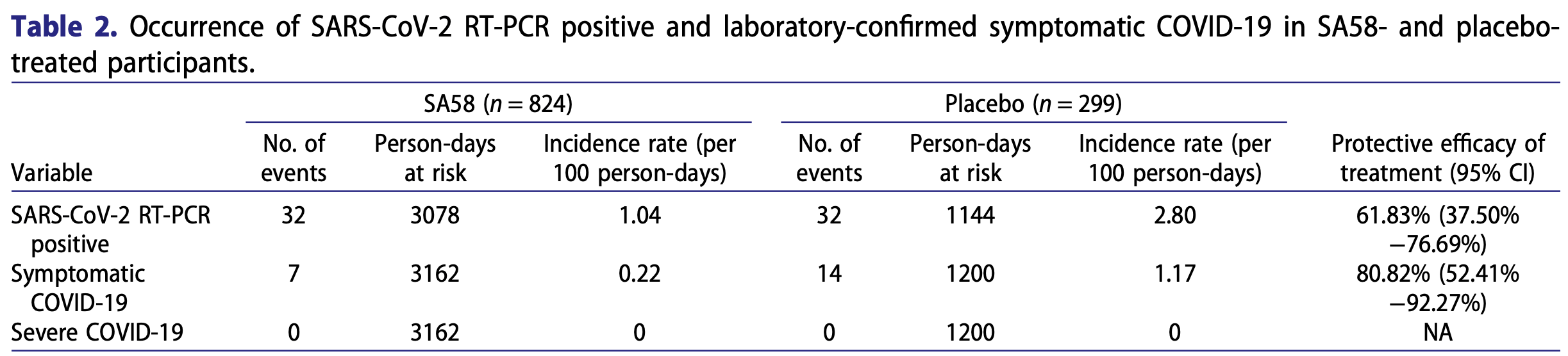
RCT 1,222 healthy adult workers in China showing SA58 (anti-SARS-CoV-2 monoclonal antibody) nasal spray reduced symptomatic COVID-19 by 81% and SARS-COV-2 infection by 62% compared to placebo when used as post-exposure prophylaxis within 72 hours of exposure. Efficacy was significantly lower when including participants who tested positive within 24 hours of first administration, suggesting SA58 is less effective once infection is established.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments4.
|
risk of symptomatic case, 80.8% lower, RR 0.19, p < 0.001, treatment 824, control 299.
|
|
risk of case, 61.8% lower, RR 0.38, p < 0.001, treatment 824, control 299.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Si et al., Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study — Hohhot City, Inner Mongolia Autonomous Region, China, 2022, China CDC Weekly, doi:10.46234/ccdcw2023.040.
2.
Song et al., Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study, Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806.
Song et al., 25 May 2023, Single Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, median age 46.0, 12 authors, study period 26 November, 2022 - 9 December, 2022, trial NCT05667714 (history).
Contact: yunlongcao@pku.edu.cn, yinwd@sinovac.com, 93353503@qq.com.
Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study*
Emerging Microbes & Infections, doi:10.1080/22221751.2023.2212806
Monoclonal antibodies (mAbs) and the post-exposure prophylaxis (PEP) with mAbs represent a very important public health strategy against coronavirus disease 2019 (COVID-19). This study has assessed a new Anti-SARS-COV-2 mAb (SA58) Nasal Spray for PEP against COVID-19 in healthy adults aged 18 years and older within three days of exposure to a SARS-CoV-2 infected individual. Recruited participants were randomized in a ratio of 3:1 to receive SA58 or placebo. Primary endpoints were laboratory-confirmed symptomatic COVID-19 within the study period. A total of 1222 participants were randomized and dosed (SA58, n = 901; placebo, n = 321). Median of follow-up was 2.25 and 2.79 days for SA58 and placebo, respectively. Adverse events occurred in 221 of 901 (25%) and 72 of 321 (22%) participants with SA58 and placebo, respectively. All adverse events were mild in severity. Laboratory-confirmed symptomatic COVID-19 developed in 7 of 824 participants (0.22 per 100 person-days) in the SA58 group vs. 14 of 299 (1.17 per 100 person-days) in the placebo group, resulting in an estimated efficacy of 80.82% (95%CI 52.41% -92.27%). There were 32 SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) positives (1.04 per 100 person-days) in the SA58 group vs. 32 (2.80 per 100 person-days) in the placebo group, resulting in an estimated efficacy of 61.83% (95%CI 37.50%-76.69%). A total of 21 RT-PCR positive samples were sequenced and all were the Omicron variant BF.7. In conclusion, SA58 Nasal Spray showed favourable efficacy and safety in preventing symptomatic COVID-19 or SARS-CoV-2 infection in adults who had exposure to SARS-CoV-2 within 72 h.
Disclosure statement XX and YC are the inventors of the provisional patent applications for the anti-SARS-CoV-2 monoclonal antibody (SA58). XX and YC are founders of Singlomics Biopharmaceuticals. SA58 have been transferred to Sinovac Biotech for clinical development. GZ, JY, XM, and WY are employees of Sinovac Biotech. JL and XL are employees of Sinovac Life Sciences. All other authors declare no competing interests.
Data sharing De-identified individual participant-level data will be available upon written request to the corresponding author following publication.
References
Brunell, Ross, Miller, Prevention of varicella by zoster immune globulin, N Engl J Med
Cao, Jian, Wang, Imprinted SARS-CoV-2 humoral immunity induces convergent omicron RBD evolution, Nature
Cao, Jian, Zhang, Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents, Cell Rep
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Ikematsu, Hayden, Kawaguchi, Baloxavir marboxil for prophylaxis against influenza in household contacts, N Engl J Med
Kuhar, Henderson, Struble, Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis
Lauer, Grantz, Bi, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med
Redeker, Mosley, Gocke, Hepatitis B immune globulin as a prophylactic measure for spouses exposed to acute type B hepatitis, N Engl J Med
Si, Li, Safety and effectiveness of SA58 nasal spray against COVID-19 infection in medical personnel: an open-label, blank-controlled studyhohhot city, Inner Mongolia autonomous region, China, China CDC Weekly
Slifka, Amanna, Passive immunization
Strohl, Ku, An, Passive immunotherapy against SARS-CoV-2: from plasma-based therapy to single potent antibodies in the race to stay ahead of the variants, BioDrugs
Walker, Whittaker, Watson, The impact of COVID-19 and strategies for mitigation and suppression in low-and middle-income countries, Science
Xin, Wang, Feng, Transmission dynamics of SARS-CoV-2 omicron variant infections in Hangzhou, Zhejiang, China, January, Int J Inf Dis
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1080/22221751.2023.2212806",
"ISSN": [
"2222-1751"
],
"URL": "http://dx.doi.org/10.1080/22221751.2023.2212806",
"alternative-id": [
"10.1080/22221751.2023.2212806"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-01-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2023-05-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2023-05-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2023-05-25"
}
],
"author": [
{
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China"
}
],
"family": "Song",
"given": "Rui",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Sinovac Biotech Co., Ltd., Beijing, People’s Republic of China"
}
],
"family": "Zeng",
"given": "Gang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9696-2460",
"affiliation": [
{
"name": "Sinovac Biotech Co., Ltd., Beijing, People’s Republic of China"
}
],
"authenticated-orcid": false,
"family": "Yu",
"given": "Jianxing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sinovac Biotech Co., Ltd., Beijing, People’s Republic of China"
}
],
"family": "Meng",
"given": "Xing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China"
}
],
"family": "Chen",
"given": "Xiaoyou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sinovac Life Sciences Co., Ltd., Beijing, People’s Republic of China"
}
],
"family": "Li",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomedical Pioneering Innovation Center (BIOPIC), Peking University, Beijing, People’s Republic of China"
},
{
"name": "Changping Laboratory, Beijing, People’s Republic of China"
}
],
"family": "Xie",
"given": "Xiaoliang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sinovac Life Sciences Co., Ltd., Beijing, People’s Republic of China"
}
],
"family": "Lian",
"given": "Xiaojuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China"
}
],
"family": "Zhang",
"given": "Zhiyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomedical Pioneering Innovation Center (BIOPIC), Peking University, Beijing, People’s Republic of China"
},
{
"name": "Changping Laboratory, Beijing, People’s Republic of China"
}
],
"family": "Cao",
"given": "Yunlong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sinovac Biotech Co., Ltd., Beijing, People’s Republic of China"
}
],
"family": "Yin",
"given": "Weidong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China"
}
],
"family": "Jin",
"given": "Ronghua",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct05667714",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Emerging Microbes & Infections",
"container-title-short": "Emerging Microbes & Infections",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2023,
5,
9
]
],
"date-time": "2023-05-09T04:11:21Z",
"timestamp": 1683605481000
},
"deposited": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T20:08:20Z",
"timestamp": 1685045300000
},
"indexed": {
"date-parts": [
[
2023,
5,
26
]
],
"date-time": "2023-05-26T04:25:49Z",
"timestamp": 1685075149101
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
5,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T00:00:00Z",
"timestamp": 1684972800000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/22221751.2023.2212806",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2023,
5,
25
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
25
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMoa1915341",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"key": "CIT0002",
"unstructured": "WHO Expert Consultation on Rabies: third report. [Internet]. World Health Organization. 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/272364/9789241210218-eng.pdf."
},
{
"key": "CIT0003",
"unstructured": "Kuhar DT, Henderson DK, Struble KA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis [Pamphlet (or booklet)]. 25/2013 Update. 2018."
},
{
"DOI": "10.1056/NEJM197511202932101",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.1056/NEJM196905292802201",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"DOI": "10.1016/B978-0-323-35761-6.00008-0",
"doi-asserted-by": "publisher",
"key": "CIT0006"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1126/science.abc0035",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"key": "CIT0009",
"unstructured": "World Health Organization. WHO Coronavirus (COVID-19) Dashboard 2022. Available from: https://covid19.who.int/"
},
{
"DOI": "10.1007/s40259-022-00529-7",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"key": "CIT0011",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) Drugs: U.S. Food and Drug Administration; 2023 [updated March 10; cited 2023 March 15]. Available from: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs"
},
{
"key": "CIT0012",
"unstructured": "U.S. Food and Drug Administration. Emergency use authorizations for drugs and Non-vaccine biological products: U.S. Food and Drug Administration; 2023 [updated March 10; cited 2023 March 15]. Available from: https://www.fda.gov/drugs/emergency-preparedness-drugs/emergency-use-authorizations-drugs-and-non-vaccine-biological-products"
},
{
"DOI": "10.1016/j.celrep.2022.111845",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"author": "Cao Y",
"first-page": "521",
"issue": "7948",
"journal-title": "Nature",
"key": "CIT0014",
"volume": "614",
"year": "2023"
},
{
"DOI": "10.46234/ccdcw2023.040",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"key": "CIT0016",
"unstructured": "The State Council PRC. ‘New Tens’ for the control and prevention of COVID-19. 2022 Dec 7. The State Council, P.R. China, 2022. Available from: http://www.nhc.gov.cn/xcs/gzzcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml."
},
{
"author": "National Health commission of the people’s republic of China",
"key": "CIT0017",
"volume-title": "Protocol for prevention and control of COVID-19",
"year": "2022"
},
{
"key": "CIT0018",
"unstructured": "NIH National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 5.0. 2017. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0."
},
{
"DOI": "10.1038/s41586-021-04385-3",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.7326/M20-0504",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1016/j.ijid.2022.10.033",
"doi-asserted-by": "publisher",
"key": "CIT0021"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/22221751.2023.2212806"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Drug Discovery",
"General Medicine",
"Immunology",
"Microbiology",
"Parasitology",
"Epidemiology"
],
"subtitle": [],
"title": "Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study*",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "12"
}
