Bromhexine Hydrochloride Prophylaxis of COVID-19 for Medical Personnel: A Randomized Open-Label Study
et al., Interdisciplinary Perspectives on Infectious Diseases, doi:10.1155/2022/4693121, NCT04405999, Mar 2021 (preprint)
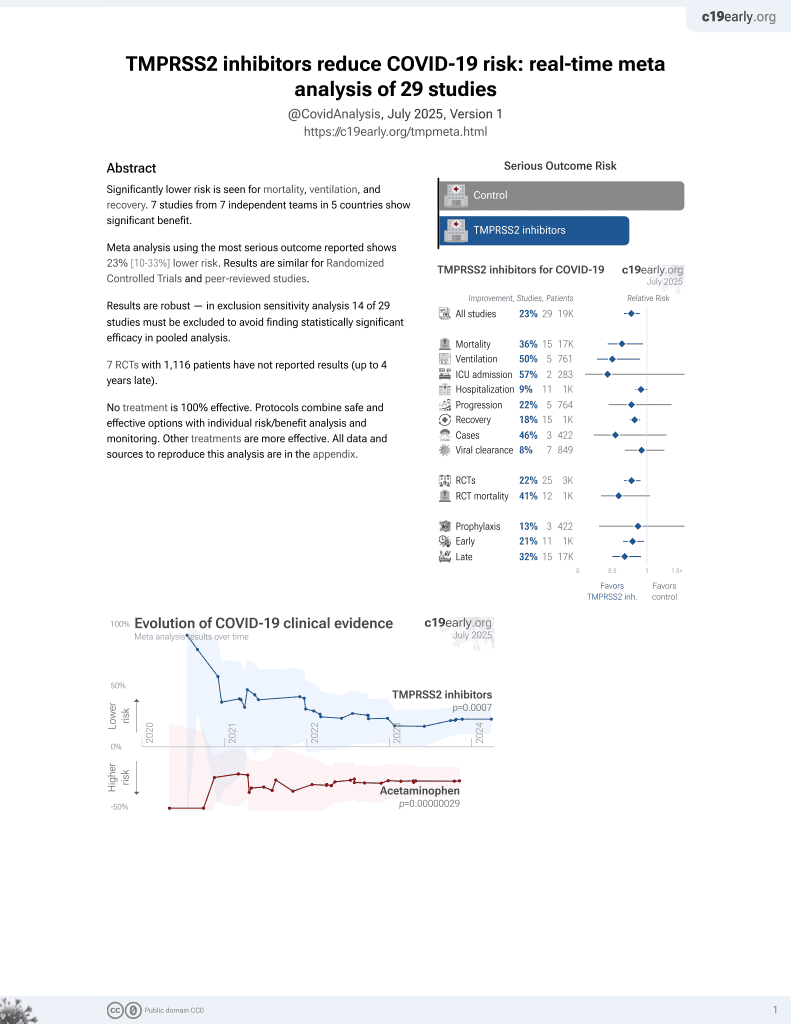
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
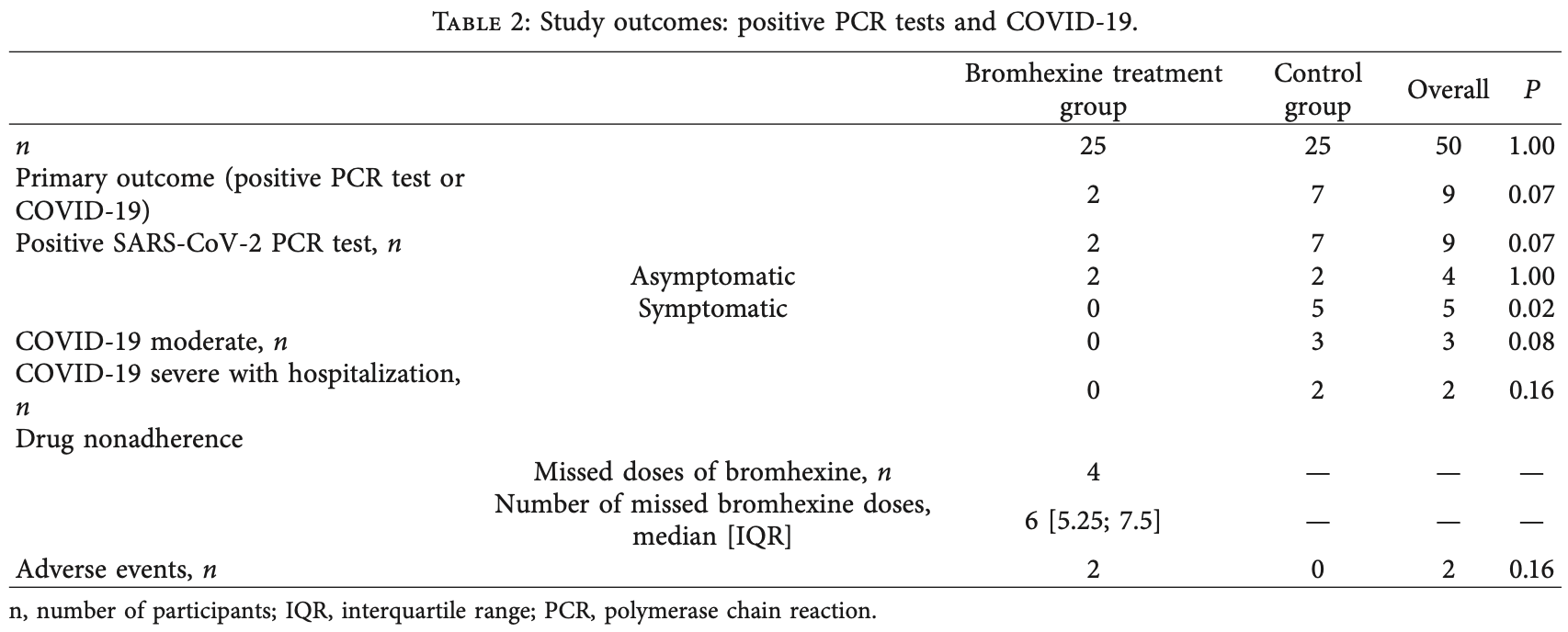
Small prophylaxis RCT with 25 treatment and 25 control health care workers, showing lower PCR+, symptomatic cases, and hospitalization with treatment, although not statistically significant with the small sample size.
Study covers TMPRSS2 inhibitors and bromhexine.
|
risk of hospitalization, 80.0% lower, RR 0.20, p = 0.49, treatment 0 of 25 (0.0%), control 2 of 25 (8.0%), NNT 12, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of symptomatic case, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 25 (0.0%), control 5 of 25 (20.0%), NNT 5.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of case, 71.4% lower, RR 0.29, p = 0.14, treatment 2 of 25 (8.0%), control 7 of 25 (28.0%), NNT 5.0, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mikhaylov et al., 8 Mar 2021, Randomized Controlled Trial, Russia, peer-reviewed, 8 authors, study period 13 May, 2020 - 25 July, 2020, trial NCT04405999 (history).
Contact: e.mikhaylov@almazovcentre.ru.
Bromhexine Hydrochloride Prophylaxis of COVID-19 for Medical Personnel: A Randomized Open-Label Study
doi:10.1101/2021.03.03.21252855
Background: Bromhexine hydrochloride has been suggested as a TMPRSS2 protease blocker that precludes the penetration of SARS-CoV-2 into cells. We aimed to assess the preventive potential of regular bromhexine hydrochloride intake for COVID-19 risk reduction in medical staff actively involved in the evaluation and treatment of patients with confirmed or suspected SARS-CoV-2 infection.
Methods : In a single-center randomized open-label study, medical staff managing patients with suspected and confirmed COVID-19 were enrolled and followed up for 8 weeks. The study began at the initiation of COVID-19 management in the clinic. The study was prematurely terminated after the enrollμent of 50 participants without a history of SARS-CoV-2 infection: 25 were assigned to bromhexine hydrochloride treatment (8 mg 3 times per day), and 25 were controls. The composite primary endpoint was a positive nasopharyngeal swab polymerase chain reaction (PCR) test for SARS-CoV-2 or signs of clinical infection within 28 days and at week 8. Secondary endpoints included: time from the first contact with a person with COVID-19 to the appearance of respiratory infection symptoms; the number of days before a first positive SARS-CoV-2 test; the number of asymptomatic participants with a positive nasopharyngeal swab test; the number of symptomatic COVID-19 cases; adverse events. Results: The rate of the combined primary endpoint did not differ significantly between the active treatment group (2/25 [8%]) and control group (7/25 [28%]); P=0.07. A fewer number of participants developed symptomatic COVID-19 in the treatment group compared to controls (0/25 vs 5/25; P = 0.02).
Conclusion: Although the study was underpowered, it showed that Bromhexine hydrochloride prophylaxis was associated with a reduced rate of symptomatic COVID-19. The prophylactic treatment was not associated with a lower combined primary endpoint rate, a positive swab PCR test, or NCT04405999
AUTHOR CONTRIBUTIONS .
Control group Overall
References
Ansarin, Tolouian, Ardalan, Taghizadieh, Varshochi et al., Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial, BioImpacts
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med
Boulware, Pullen, Bangdiwala, Pastick, Lofgren et al., A Randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19, N Engl J Med
Dyer, Covid-19: cases rise in Russia as health workers pay the price for PPE shortage, BMJ
Gandhi, Lynch, Rio, Mild or moderate Covid-19, N Engl J Med
Glowacka, Bertram, Müller, Allen, Soilleux et al., Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J Virol
Habtemariam, Nabavi, Ghavami, Cismaru, Berindan-Neagoe et al., Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARS-CoV-2 infection based on its action on the transmembrane serine protease 2, Pharmacol Res
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control, J Clin Med
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Li, Sun, Zhang, Zheng, Jiang et al., Bromhexine hydrochloride tablets for the treatment of moderate COVID-19: an open-label randomized controlled pilot study, Clin Transl Sci
Lucas, Heinlein, Kim, Lucas, Heinlein et al., The androgenregulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis, Cancer Discovery
Marty, Chen, Verrill, How to obtain a nasopharyngeal swab specimen, N Engl J Med
Matsuyama, Nagata, Shirato, Kawase, Takeda et al., Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2, J Virol
Mcmichael, Currie, Clark, Pogosjans, Kay et al., Epidemiology of Covid-19 in a long-term care facility in King County, Washington, N Engl J Med
Mikhaylov, Lyubimtseva, Vakhrushev, Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: a randomized open-label study, MedRxiv
Nguyen, Drew, Graham, Joshi, Guo et al., Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study, Lancet Public Health
Qaseem, Yost, Etxeandia-Ikobaltzeta, Miller, Abraham et al., Update alert: should clinicians use chloroquine or hydroxychloroquine alone or in combination with azithromycin for the prophylaxis or treatment of COVID-19? Living practice points from the American College of Physicians, Annals of internal medicine
Shulla, Heald-Sargent, Subramanya, Zhao, Perlman et al., A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry, J Virol
Simmons, Gosalia, Rennekamp, Reeves, Diamond et al., Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry, Proc Natl Acad Sci
Stepanov, Lierz, Bromhexine hydrochloride: potential approach to prevent or treat early stage COVID-19, J Infect Dis Epidemiol
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
DOI record:
{
"DOI": "10.1155/2022/4693121",
"ISSN": [
"1687-7098",
"1687-708X"
],
"URL": "http://dx.doi.org/10.1155/2022/4693121",
"abstract": "<jats:p>Background. Bromhexine hydrochloride has been suggested as a TMPRSS2 protease blocker that precludes the penetration of SARS-CoV-2 into cells. We aimed to assess the preventive potential of regular bromhexine hydrochloride intake for COVID-19 risk reduction in medical staff actively involved in the evaluation and treatment of patients with confirmed or suspected SARS-CoV-2 infection. Methods. In a single-centre randomized open-label study, medical staff managing patients with suspected and confirmed COVID-19 were enrolled and followed up for 8 weeks. The study began at the initiation of COVID-19 management in the clinic. The study was prematurely terminated after the enrollment of 50 participants without a history of SARS-CoV-2 infection: 25 were assigned to bromhexine hydrochloride treatment (8 mg 3 times per day), and 25 were controls. The composite primary endpoint was a positive nasopharyngeal swab polymerase chain reaction (PCR) test for SARS-CoV-2 or signs of clinical infection within 28 days and at week 8. Secondary endpoints included time from the first contact with a person with COVID-19 to the appearance of respiratory infection symptoms; the number of days before a first positive SARS-CoV-2 test; the number of asymptomatic participants with a positive nasopharyngeal swab test; the number of symptomatic COVID-19 cases; and adverse events. Results. The rate of the combined primary endpoint did not differ significantly between the active treatment group (2/25 [8%]) and control group (7/25 [28%]); <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.07</mn>\n </math>\n </jats:inline-formula>. A fewer number of participants developed symptomatic COVID-19 in the treatment group compared to controls (0/25 vs. 5/25; <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.02</mn>\n </math>\n </jats:inline-formula>). Conclusion. Although the study was underpowered, it showed that Bromhexine hydrochloride prophylaxis was associated with a reduced rate of symptomatic COVID-19. The prophylactic treatment was not associated with a lower combined primary endpoint rate, a positive swab PCR test, or COVID-19 (ClinicalTrials.gov number, NCT04405999).</jats:p>",
"alternative-id": [
"4693121",
"4693121"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6553-9141",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Mikhaylov",
"given": "Evgeny N.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8651-7777",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Lyubimtseva",
"given": "Tamara A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0116-7753",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Vakhrushev",
"given": "Aleksandr D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1152-9148",
"affiliation": [
{
"name": "Department of Anesthesiology, Intensive Care, Pain Management and Palliative Care, Marienkrankenhaus Soest, Soest, Germany"
}
],
"authenticated-orcid": true,
"family": "Stepanov",
"given": "Dmitry",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2334-1663",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Lebedev",
"given": "Dmitry S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"family": "Vasilieva",
"given": "Elena Yu.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8169-7812",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Konradi",
"given": "Alexandra O.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2929-0980",
"affiliation": [
{
"name": "Almazov National Medical Research Centre, Saint-Petersburg, Russia"
}
],
"authenticated-orcid": true,
"family": "Shlyakhto",
"given": "Evgeny V.",
"sequence": "additional"
}
],
"container-title": "Interdisciplinary Perspectives on Infectious Diseases",
"container-title-short": "Interdisciplinary Perspectives on Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
29
]
],
"date-time": "2022-01-29T16:20:08Z",
"timestamp": 1643473208000
},
"deposited": {
"date-parts": [
[
2022,
1,
29
]
],
"date-time": "2022-01-29T16:20:16Z",
"timestamp": 1643473216000
},
"editor": [
{
"affiliation": [],
"family": "Tharmalingam",
"given": "Jayaraman",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/501100012190",
"award": [
"075-15-2020-901"
],
"doi-asserted-by": "publisher",
"name": "Federal Target Program"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
12
]
],
"date-time": "2023-08-12T19:15:05Z",
"timestamp": 1691867705559
},
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2022,
1,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
29
]
],
"date-time": "2022-01-29T00:00:00Z",
"timestamp": 1643414400000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/ipid/2022/4693121.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ipid/2022/4693121.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/ipid/2022/4693121.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-7",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2022,
1,
29
]
]
},
"published-print": {
"date-parts": [
[
2022,
1,
29
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"DOI": "10.3390/jcm9041225",
"doi-asserted-by": "publisher",
"key": "1"
},
{
"DOI": "10.1001/jama.2020.12839",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"DOI": "10.1056/nejmcp2009249",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1056/nejmcp2009575",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.1056/nejmoa2016638",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"DOI": "10.7326/m20-3862",
"doi-asserted-by": "publisher",
"key": "6"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1128/jvi.02232-10",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"article-title": "Bromhexine hydrochloride: potential approach to prevent or treat early stage COVID-19",
"author": "D. Stepanov",
"first-page": "135",
"journal-title": "J Infect Dis Epidemiol",
"key": "9",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1128/jvi.01542-10",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1128/jvi.02062-10",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1073/pnas.0505577102",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.1158/2159-8290.cd-13-1010",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.1016/j.phrs.2020.104853",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.34172/bi.2020.27",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"article-title": "Interim-20-ID-01: standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19)",
"author": "Council of State and Territorial Epidemiologists",
"key": "16",
"year": "2020"
},
{
"DOI": "10.1016/S2468-2667(20)30164-X",
"doi-asserted-by": "publisher",
"key": "17"
},
{
"DOI": "10.1136/bmj.m1975",
"doi-asserted-by": "publisher",
"key": "18"
},
{
"DOI": "10.15585/mmwr.mm6915e6",
"article-title": "Characteristics of health care personnel with COVID-19—United States",
"author": "CDC COVID-19 Response Team",
"doi-asserted-by": "crossref",
"first-page": "477",
"issue": "15",
"journal-title": "Morbidity and Mortality Weekly Report",
"key": "19",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1056/nejmoa2005412",
"doi-asserted-by": "publisher",
"key": "20"
},
{
"DOI": "10.1056/nejmvcm2010260",
"doi-asserted-by": "publisher",
"key": "21"
},
{
"DOI": "10.1111/cts.12881",
"doi-asserted-by": "publisher",
"key": "22"
},
{
"article-title": "Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: a randomized open-label study",
"author": "E. N. Mikhaylov",
"journal-title": "MedRxiv",
"key": "23",
"volume": "2021",
"year": "2021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/ipid/2022/4693121/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Microbiology (medical)",
"Microbiology",
"Parasitology"
],
"subtitle": [],
"title": "Bromhexine Hydrochloride Prophylaxis of COVID-19 for Medical Personnel: A Randomized Open-Label Study",
"type": "journal-article",
"volume": "2022"
}