RECOVER: Phase 2 Randomized, Double-Blind Trial TREating Hospitalized Patients With COVID-19 With Camostat MesilatE, a TMPRSS2 Inhibitor
et al., NCT04470544, RECOVER, NCT04470544, Mar 2024
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
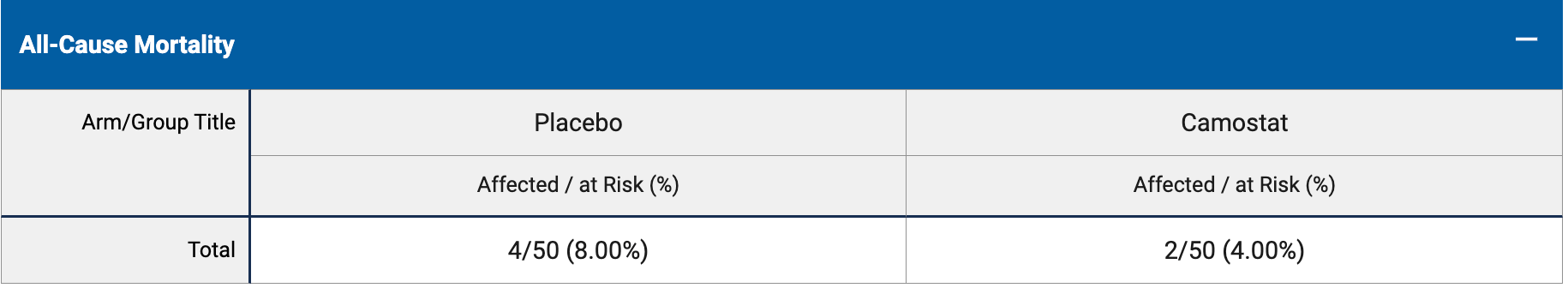
RCT 100 patients showing no significant difference with camostat. Results are currently unclear - different mortality numbers were provided for all-cause mortality and mortality rate (2/50 vs. 3/46 for the treatment group at 28 days, with the 28 day all-cause mortality result removed in an updated submission). The main outcome measures appear to be different due to only including patients that submitted day 28 outcome data.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of death, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 50 (6.0%), control 4 of 50 (8.0%), NNT 50, day 56.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bryce et al., 28 Mar 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04470544 (history) (RECOVER).
Contact: Nguyen.Vy@mayo.edu, ClinicalResearch@tmcaz.com, Vimoktayon.Torsak@mayo.edu, Pecenka.Stacey@mayo.edu.